Abstract
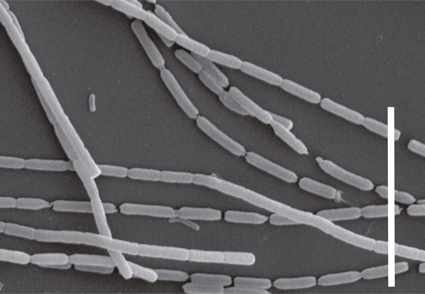
Two Leptolyngbya-like cyanobacterial strains were isolated from two marine benthic environments on the Brazilian coast. These strains were cultured and characterized based on their morphology, molecular, and ultrastructural data. The two taxa were identified mainly by 16S rRNA gene phylogeny and 16S-23S ITS secondary structures since their morphology is similar to members of Leptolyngbya senso lato. The phylogenetic analysis indicated that our strains belong to Euryhalinema genus (Leptolyngbyaceae), with one strain close to type species E. mangrovii AP9F (MK402979), and the other described as a new species, E. epiphyticum sp. nov. Morphologically, both strains form green mats, had trichomes without sheath, and their thylakoid disposition is the same as described for Leptolyngbyaceae. The secondary structures Box B and D1-D1’ of the internal transcribed spacer (16S–23S ITS) also corroborated our proposal of the new species E. epiphyticum. These findings constitute the first description of a new-to-science species for this genus outside Indian marine environments. Also, it expands the knowledge on Euryhalinema systematic.
References
Becerra-Absalón, I., Johansen, J.R., Muñoz-Martín, M.A. & Montejano, G. (2018) Chroakolemma gen. nov. (Leptolyngbyaceae, Cyanobacteria) from soil biocrusts in the semi-desert Central Region of Mexico. Phytotaxa 367: 201–218. https://doi.org/10.11646/phytotaxa.367.3.1
Birnboim, H.C. & Doly, J. (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Research 7: 1513–1518. https://doi.org/10.1093/nar/7.6.1513
Caires, T.A., Sant’Anna, C.L., Nunes, J.M.C. (2019) Biodiversity of benthic filamentous cyanobacteria in tropical marine environments of Bahia State, Northeastern Brazil. Brazilian Journal of Botany 42: 149–170. https://doi.org/10.1007/s40415-019-00517-2
Castenholz, R.W. (1988) Culturing methods for cyanobacteria. Methods Enzymol 167: 68–93. https://doi.org/10.1016/0076-6879(88)67006-6
Chakraborty, S., Maruthanayagam, V., Achari, A., Pramanik, A., Jaisankar, P. & Mukherjee, J. (2019) Euryhalinema mangrovii gen. nov., sp. nov. and Leptoelongatus litoralis gen. nov., sp. nov. (Leptolyngbyaceae) isolated from an Indian mangrove forest. Phytotaxa 422 (1): 58–74. https://doi.org/10.11646/phytotaxa.422.1.4
Dvo?ák, P., Jahodá?ová, E., Hašler, P., Gusev, E., Poulí?ková, A. (2015) A new tropical cyanobacterium Pinocchia polymorpha gen. et sp. nov. derived from the genus Pseudanabaena. Fottea 15 (1): 113–120. https://doi.org/10.5507/fot.2015.010
Iteman, I., Rippka, R., de Marsac, N.T., Hergman, M. (2000) Comparison of conserved structural and regulatory domains within divergent 16S rRNA–23S rRNA spacer sequences of cyanobacteria. Microbiology 146: 1275–1286. https://doi.org/10.1099/00221287-146-6-1275
Jacinavicius, F.R., Gama, W.A.Jr., Azevedo, M.T.P. & Sant’Anna, C.L. (2012) Manual para cultivo de cianobactérias. Publicações Online do Instituto de Botânica de São Paulo, São Paulo, 32 pp.
Karnovsky, M.J. (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. Journal of Cell Biology 27: 1A–149A.
Komárek, J. (2007) Phenotype diversity of the cyanobacterial genus Leptolyngbya in the maritime Antartic. Polish Polar Research, 20 (3): 211–231.
Komárek, J. & Anagnostidis, K. (2005) Cyanoprokaryota-2. Teil/2nd part: Oscillatoriales. In: Büdel, B., Gärtner, G., Krienitz, L. & Schagerl, M. (Eds.) Süsswasserflora von Mitteleuropa 19 ? 778 2. Elsevier/Spektrum, Heidelberg, pp. 1–759.
Komárek, J., Kaštovský, J., Mareš, J., Johansen, J.R. (2014) Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 86: 295–335.
Konstantinou, D., Voultsiadou, E., Panteris, E., Zervou, S-K., Hiskia, A., Gkelis, S. (2019) Leptothoe, a new genus of marine cyanobacteria (Synechococcales) and three new species associated with sponges from the Aegean Sea. J. Phycol. 55: 882–897. https://doi.org/10.1111/jpy.12866
Lane, D.J. (1991) 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics (Stackebrandt, E., Goodfellow, M., editors). John Wiley and Sons, Chichester, United Kingdom, pp. 115–175.
Lukesová, A., Johansen, J.R., Martin, M.P., Casamatta, D.A. (2009) Aulosira bohemensis sp. nov.: further phylogenetic uncertainty at the base of Nostocales (Cyanobacteria). Phycologia 48: 118–129. https://doi.org/10.2216/08-56.1
Mai, T., Johansen, J., Pietrasiak, N., Bohunická, M., Martin, M.P. (2018) Polyphasic characterization of four species of Pseudanabaena (Oscillatoriales, Cyanobacteria) from China and insights into polyphyletic divergence within the Pseudanabaena genus. Phytotaxa, 365 (1): 001–059.
Mau, B., Newton, M.A., Larget, B., 1999. Bayesian phylogenetic inference via Markov chain Monte Carlo methods. Biometrics 55: 1–12. https://doi.org/10.1111/j.0006-341X.1999.00001.x
Neilan, B., A., Jacobs, D., DelDot, T., Blackall, L.L., Hawkins, P.R., Cox, P.T., Goodman, A.E. (1997) rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. International journal of systematic bacteriology 47: 693–697. https://doi.org/10.1099/00207713-47-3-693
Nylander, J.A.A. (2008) MrModeltest 2.3. Program Distributed by the Author. Evolutionary Biology Centre, Uppsala University.
Osorio–Santos, K., Pietrasiak, N., Bohunická, M., Miscoe, L.H., Ková?ik, L., Martin, M.P. Johansen, J.R. (2014) Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): taxonomically recognizing cryptic diversification. European journal of phycology 49: 450–470. https://doi.org/10.1080/09670262.2014.976843
Posada, D. & Crandall, K. (1998) MODELTEST: Testing the Model of DNA Substitution. Bioinformatics 14: 817–818. https://doi.org/10.1093/bioinformatics/14.9.817
R Core Team. (2017) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [http://www.R-project.org/]
Radzi, R., Muangmai, N., Broady, P., Omar, W.M.W., Lavoue, S., Convey, P. & Merican, F. (2019) Nodosilinea signiensis sp. nov. (Leptolyngbyaceae, Synechococcales), a new terrestrial cyanobacterium isolated from mats collected on Signy Island, South Orkney Islands, Antarctica. PLoS ONE 14 (11): e0224395. https://doi.org/10.1371/journal.pone.0224395
Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M. & Stanier, R.Y. (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Journal of General Microbiology 111: 1–61. https://doi.org/10.1099/00221287-111-1-1
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61: 539– 847. https://doi.org/10.1093/sysbio/sys029
Sambrook, J.F. & Russell, D.W. (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York, 2100 pp.
Sciuto, K. & Moro, I. (2016) Detection of the new cosmopolitan genus Thermoleptolyngbya (Cyanobacteria, Leptolyngbyaceae) using the 16S rRNA gene and 16S-23S ITS region. Molecular Phylogenetics and Evolution 105: 15–35. https://doi.org/10.1016/j.ympev.2016.08.010
Sciuto, K., Moschin, E. & Moro, I. (2017) Cryptic cyanobacterial diversity in the giant cave (Trieste, Italy): the new genus Timaviella (Leptolyngbyaceae). Cryptogamie Algologie 38 (4): 285–323. https://doi.org/10.7872/crya/v38.iss4.2017.285
Song, G.F., Jiang, Y.G. & Li, R. (2015) Scytolyngbya timoleontis, gen. et sp. nov. (Leptolyngbyaceae, Cyanobacteria): a novel false branching Cyanobacteria from China. Phytotaxa 224: 72–84. https://doi.org/10.11646/phytotaxa.224.1.5
Stamatakis, A. (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Stamatakis, A., Hoover, P. & Rougemont, J. (2008) A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758–771. https://doi.org/10.1080/10635150802429642
Taton, A., Grubisic, S., Brambilla, E., De Wit, R. & Wilmotte, A. (2003) Cyanobacterial diversity in natural and artificial microbial mats of Lake Fryxell (McMurdo Dry Valleys, Antarctica): a morphological and molecular approach. Applied Environmental Microbiology 69: 5157–69. https://doi.org/10.1128/AEM.69.9.5157-5169.2003
Ulcay, S., Ta?kin, E., Kurt, O. & Öztürk, M. (2015) Marine benthic cyanobacteria in Northern Cyprus (Eastern Mediterranean Sea). Turkish Journal of Botany 39: 173– 188. https://doi.org/10.3906/bot-1311-52
Vaz, M.G.M.V., Genuário, D.B., Andreote, A.P.D., Malone, C.F.S., Sant´Anna, C.L., Barbiero, L. & Fiore, M.F. (2015) Pantanalinema gen. nov. and Alkalinema gen. nov.: two novel pseudanabaenacean genera (Cyanobacteria) isolated from saline-alkaline lakes. International Journal of Systematic and Evolutionary Microbiology 65: 298–308. https://doi.org/10.1099/ijs.0.070110-0
Zammit, G., Billi, D. & Albertano, P. (2012) The subaerophytic cyanobacterium Oculatella subterranea (Oscillatoriales, Cyanophyceae) gen. et sp. nov.: a cytomorphological and molecular description. European Journal of Phycology 47: 341–354. https://doi.org/10.1080/09670262.2012.717106
Zhou, W., Ding, D., Yang, Q., Ahmad, M., Zhang, Y., Lin, X., Zhang, Y., Ling, J. & Dong, J. (2018) Marileptolyngbya sina gen. nov., sp. nov. and Salileptolyngbya diazotrophicum gen. nov., sp. nov. (Synechococcales, Cyanobacteria), species of cyanobacteria isolated from a marine ecosystem. Phytotaxa 383: 75–92. https://doi.org/10.11646/phytotaxa.383.1.4
Zubia, M., Turquet, J. & Golubic, S. (2016) Benthic cyanobacterial diversity of iles eparses [Scattered islands] in the Mozambique channel. Acta Oecologica 72: 21–32. https://doi.org/10.1016/j.actao.2015.09.004
Zubia, M., Vieira, C., Palinska, K.A., Roué, M., Gaertner, J.-C., Zloch, I. & Golubic, S. (2019) Benthic cyanobacteria on coral reefs of Moorea Island (French Polynesia): diversity response to habitat quality. Hydrobiologia 843: 61–78. https://doi.org/10.1007/s10750-019-04029-8
Zuker, M. (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research 31: 3406–3415. https://doi.org/10.1093/nar/gkg595