Abstract
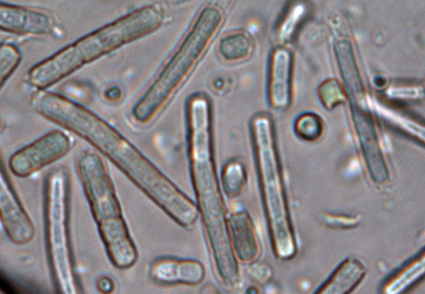
Timaviella Sciuto & Moro is a recently established cryptic genus of cyanobacteria separated from the morphologically close Leptolyngbya due to clear differences in the 16S rRNA gene sequence and the 16S-23S ITS region secondary structure. Conducting research on biological soil crusts in coastal ecotopes of Ukraine and Germany, we repeatedly observed thin filamentous cyanobacteria morphologically corresponding to the common terrestrial species Leptolyngbya edaphica (Elenkin) Anagnostidis & Komárek. Molecular data based on 16S rRNA gene sequence comparison of the original strains of the morphospecies indicated unambiguous assignment to the genus Timaviella. Based on this finding, we proposed the new nomenclatural combination Timaviella edaphica (Elenkin) O.M. Vynogr. & Mikhailyuk in our previous publication. Deeper molecular study of the four original strains which were morphologically identified as T. edaphica based on the 16S rRNA gene concatenated with the 16S-23S ITS region and 16S-23S ITS secondary structure analysis showed that they are not identical. Three of them (isolated from biocrusts of Black Sea coast and forest path near Kyiv, Ukraine) had high similarity both in 16S rRNA (99.7–100%) and 16S-23S ITS (99.8–100%) hence actually representing T. edaphica. The strain Us-6-3 isolated from biocrusts on sand dunes of Usedom Island in the Baltic Sea, Germany, differs both from original strains of T. edaphica and all published Timaviella species in 16S rRNA gene sequence identity, as well as in sequence and structure of the 16S-23S ITS region. Here we describe Timaviella dunensis sp. nov. and give an expanded description of T. edaphica based on morphological and molecular features. A tabular review of Timaviella species with data on their phenotypic and genotypic features, ecology and distribution is included.
References
Anagnostidis, K. & Komárek, J. (1988) Modern approach to the classification system of cyanophytes. 3. Oscillatoriales. Algological Studies 50–53: 327–472.
Becerra-Absalón, I., Johansen, J.R., Osorio-Santos, K., Montejano, G. (2020) Two new Oculatella (Oculatellaceae, Cyanobacteria) species in soil crusts from tropical semiarid uplands of México. Fottea, Olomouc 20: 160–170. https://doi.org/10.5507/fot.2020.010
Bischoff, H.W. & Bold, H.C. (1963) Phycological studies IV. Some soil algae from Enchanted Rock and related algal species. University of Texas Publications 6318, Austin, 95 pp.
Byun, Y. & Han, K. (2009) PseudoViewer3: generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 25 (11): 1435–1437. https://doi.org/10.1093/bioinformatics/btp252
Casamatta, D.A.,Vis, M.L. & Sheath, R.G. (2003) Cryptic species in cyanobacterial systematics: a case study of Phormidium retzii (Oscillatoriales) using RAPD molecular markers and 16S rDNA sequence data. Aquatic Botany 77: 295–309. https://doi.org/10.1016/j.aquabot.2003.08.005
Davydov, D., Shalygin, S. & Vilnet, A. (2020) New cyanobacterium Nodosilinea svalbardensis sp. nov. (Prochlorotrichaceae, Synechococcales) isolated from alluvium in Mimer river valley of the Svalbard archipelago. Phytotaxa 442: 61–79. https://doi.org/10.11646/phytotaxa.442.2
Dvo?ák, P., Hindák, F., Hašler, P., Hindáková, A. & Poulí?ková, A. (2014) Morphological and molecular studies of Neosynechococcus sphagnicola gen. et sp. nov.(Cyanobacteria, Synechococcales). Phytotaxa 170: 024–034. https://doi.org/10.11646/phytotaxa.170.1.3
Dvo?ák, P., Poulí?ková, A., Hašler, P., Belli, M., Casamatta, D. & Papini, A. (2015) Species concepts and speciation factors in cyanobacteria, with connection to the problems of diversity and classification. Biodiversity and Conservation 24: 739–757. https://doi.org/10.1007/s10531-015-0888-6
Elenkin, A.A. (1949) Monographia Algarum Cyanophycearum Aquidulcium et Terrestrium in finibus URSS inventarum, Spec. pt 2. AN USSR Press, Moscow; Leningrad [Rus.]
Ellis, E.A. (2006) Corrected formulation for Spurr low viscosity embeddig medium using the replacement Epoxide ERL 4221. Microscopy and Microanalysis 12: 288–289. https://doi.org/10.1017/S1431927606062660
Erwin, P.M. & Thacker, R.W. (2008) Cryptic diversity of the symbiotic cyanobacterium Synechococcus spongiarum among sponge host. Molecular Ecology 17: 2937–2947. https://doi.org/10.1111/j.1365-294X.2008.03808.x
González-Reséndiz, L., Johansen, J.R., Léon-Tejera, H., Sanchez, L., Segal-Kischinevzky, C., Escobar-Sánchez, V. & Morales, M. (2019) A bridge too far in naming species: a total evidence approach does not support recognition of four species in Desertifilum (cyanobacteria). Journal of Phycology 55: 898?911. https://doi.org/10.1111/jpy.12867
Guiry, M.D. & Guiry, G.M. (2021) AlgaeBase. Worldwide electronic publication, Nat. Univ. Ireland, Galway. Available from: http://www. algaebase.org (accessed 25 July 2021)
Holzinger, A., Roleda, M.Y. & Lütz, C. (2009) The vegetative arctic green alga Zygnema is insensitive to experimental UV exposure. Micron 40: 831–838. https://doi.org/10.1016/j.micron.2009.06.008
Jahodá?ová, E., Dvo?ák, P., Hašler, P., Holušová, K. & Poulí?ková, A. (2017) Elainella gen. nov.: a new tropical cyanobacterium characterized using a complex genomic approach. European Journal of Phycology 53: 39–51. https://doi.org/10.1080/09670262.2017.1362591
Jung, P., Mikhailyuk, T., Emrich, D., Baumann, K., Dultz, S. & Büdel, B. (2020) Shifting Boundaries: ecological and geographical range extension based on three new species in the cyanobacterial genera Aliterella, Cyanocohniella and Oculatella. Journal of Phycology 56: 1216–1231. https://doi.org/10.1111/jpy.13025
Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010
Klymchuk, D.O., Brown, C.S., Chapman, D.K., Vorobyova, T.V. & Martyn, G.M. (2001) Cytochemical localization of calcium in soybean root cap cells in microgravity. Advances in Space Research 27 (5): 967–972. https://doi.org/10.1016/S0273-1177(01)00160-0
Komárek, J. & Anagnostidis, K. (2005) Cyanoprokaryota. 2. Oscillatoriales. Süsswasserflora von Mitteleuropa, Bd 19/2, Elsevier Spectr., München.
Kondratyeva, N.V. (1968) Class Hormogoniophyceae. Identification manual of freshwater algae of Ukrainian SSR, Vol. 1, pt 2, Naukova Dumka Press, Kyiv [Ukr.]
Konstantinou, D., Voultsiadou, E., Panteris,E., Zervou,S.-K., Hiskia, A. & Gkelis, S. (2019) Leptothoe, a new genus of marine cyanobacteria (Synechococcales) and three new species associated with sponges from the Aegean Sea. Journal of Phycology 55: 882–897. https://doi.org/10.1111/jpy.12866
Mai, T., Johansen, J.R., Pietrasiak, N., Bohunická, M. & Martin, M.P. (2018) Revision of the Synechococcales (Cyanobacteria) through recognition of four families including Oculatellaceae fam. nov. and Trichocoleaceae fam. nov. and six new genera containing 14 species. Phytotaxa 365 (1): 1–59. https://doi.org/10.11646/phytotaxa.365.1.1
Marin, B., Nowack, E.C.M. & Melkonian, M. (2005) A plastid in the making: evidence for a second primary endosymbiosis. Protist 156: 425–432. https://doi.org/10.1016/j.protis.2005.09.001
Mesfin, M., Johansen, J.R., Pietrasiak, N. & Baldarelli, L.M. (2020) Nostoc oromo sp. nov. (Nostocales, Cyanophyceae) from Ethiopia: a new species based on morphological and molecular evidence. Phytotaxa 224 (1): 81–93. https://doi.org/10.11646/phytotaxa.433.2
Mikhailyuk, T.?., Vinogradova, O.N., Glaser, K. & Karsten, U. (2016) New taxa for the flora of Ukraine, in the context of modern approaches to taxonomy of Cyanoprokaryota/Cyanobacteria. International Journal on Algae 18 (4): 301–320. https://doi.org/10.1615/InterJAlgae.v18.i4.10
Mikhailyuk, T.I., Vinogradova, O., Glaser, K., Demchenko, E.M. & Karsten, U. (2018) Diversity of terrestrial algae of the Cape Kazantip (the Sea of Azov, Ukraine) with special reference to their phylogeny and ecology. International Journal on Algae 20: 313–338. https://doi.org/10.1615/InterJAlgae.v20.i4.10
Mikhailyuk, T., Glaser, K., Tsarenko, P., Demchenko, E. & Karsten, U. (2019) Composition of biological soil crusts from sand dunes of the Baltic Sea coast, in the context of an integrative approach to the taxonomy of microalgae and cyanobacteria. European Journal of Phycology 54: 263–290. https://doi.org/10.1080/09670262.2018.1557257
Osorio-Santos, K., Pietrasiak, N., Bohunická, M., Miscoe, L.H., Ková?ik, L., Martin, M.P. & Johansen, J.R. (2014) Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): taxonomically recognizing cryptic diversification. European Journal of Phycology 49: 450–470. https://doi.org/10.1080/09670262.2014.976843
Pietrasiak, N., Osorio-Santos, K., Shalygin, S., Martin, M.P. & Johansen, J.R. ( 2019). When is a lineage a species? A case study in Myxacorys gen. nov. (Synechococcales: Cyanobacteria) with the description of two new species from the Americas. Journal of Phycology 55: 976–996. https://doi.org/10.1111/jpy.12897
Ronquist, F. & Huelsenbeck, J.P. (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Schulz, K., Mikhailyuk, T., Dreßler, M., Leinweber, P. & Karsten, U. (2016) Biological soil crusts from coastal dunes at the Baltic Sea:cyanobacterial and algal biodiversity and relatedsoil properties. Microbial Ecology 71: 178–193. https://doi.org/10.1007/s00248-015-0691-7
Sciuto, K., Moschin, E. & Moro, I. (2017) Cryptic cyanobacterial diversity in the Giant Cave (Trieste, Italy): the new genus Timaviella (Leptolyngbyaceae). Cryptogamie, Algologie 38: 285–323. https://doi.org/10.7872/crya/v38.iss4.2017.285
Shalygin, S., Pietrasiak, N., Gomez, F., Mlewski, C., Gerard, E. & Johansen, J.R. (2018) Rivularia halophila sp. nov. (Nostocales, Cyanobacteria): the first species of Rivularia described with the modern polyphasic approach. European Journal of Phycology 53 (4): 537–548. https://doi.org/10.1080/09670262.2018.1479887
Shalygin, S., Shalygina, R., Redkina, V.V., Gargas, C.B. & Johansen, J.R. (2020) Description of Stenomitos kolaenensis and S. hiloensis sp. nov. (Leptolyngbyaceae, Cyanobacteria) with an emendation of the genus. Phytotaxa 440 (2): 72–84. https://doi.org/10.11646/phytotaxa.440.2.3
Song, G., Jiang, Y. & Li, R. (2015) Scytolyngbya timoleontis gen et sp. nov. (Leptolyngbyaceae, Cyanobacteria): a novel false branching Cyanobacteria from China. Phytotaxa 224 (1): 72–84. https://doi.org/10.11646/phytotaxa.224.1.5
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA 6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. https://doi.org/10.1093/molbev/mst197
Vaulina, E.N. (1959) On the systematic position of the soil form Plectonema puteale (Kirchn.) Hansg. Bot. Mat. Dept. Spore Plants 12: 19–22.
Vinogradova, O. & Mikhailyuk, T. (2018) On the taxonomy and nomenclature of some terrestrial taxa of Plectonema s. l. (?yanophyceae). 1. The case of Plectonema edaphicum. International Journal on Algae 20 (3): 211–224. https://doi.org/10.1615/InterJAlgae.v20.i3.10
Vinogradova, O., Kovalenko, O.V., Wasser, S.P., Nevo, E. & Weinstein-Evron, M. (1998) Species diversity gradient to darkness stress in blue-green algae/cyanobacteria: a microscale test in a prehistoric cave, Mount Carmel, Israel. Israel Journal of Plant Sciences 46: 366–378. https://doi.org/10.1080/07929978.1998.10676732
Vinogradova, O., Nevo, E. & Wasser, S.P. (2009) Algae of the Sefunim Cave (Israel): species diversity affected by light, humidity and rock stresses. International Journal on Algae 11: 99–116. https://doi.org/10.1615/InterJAlgae.v11.i2.10
Vinogradova, O., Mikhailyuk, T., Glaser, K., Holzinger, A. & Karsten, U. (2017) New species of Oculatella (Synechococcales, Cyanobacteria) from terrestrial habitats of Ukraine. Ukrainian Botanical Journal 74 (6): 509–520. https://doi.org/10.15407/ukrbotj74.06.509
Wilmotte, A., Van der Auwera, G. & De Wachter, R. (1993) Structure of the 16S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (Mastigocladus laminosus HTF’) strain PCC75 18, and phylogenetic analysis. FEBS Letters 317: 96–100. https://doi.org/10.1016/0014-5793(93)81499-P
Zuker, M. (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research 31: 3406–3416.