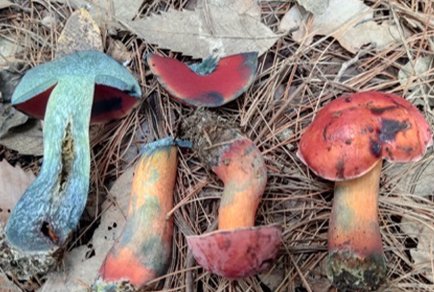Abstract
Based on molecular phylogenetic analyses of the ITS and LSU regions of nuclear DNA and morphological evidences, Rubroboletus quercicola is proposed as a new species. The newly described species is characterized by a vivid red to orangish red pileus, which is viscid when wet; a smooth to finely fibrillose, clavate stipe with a creamy white context; fusoid to fusoid-ventricose cheilocystidia and pleurocystidia; and fusiform to subfusiform caulocystidia. Morphological description and illustrations of the newly described species are given, and similarities and differences with the closely related species are discussed.
References
- Ammirati, J.F. (1985) Poisonous mushrooms of the northern United States and Canada. University of Minnesota Press, Minneapolis.
- Benjamin, D.R. (1995) Mushrooms: poisons and panaceas: a handbook for naturalists, mycologists, and physicians. W.H. Freeman and Company, New York, 422 pp.
- Chiu, W.F. (1948) The boletes of Yunnan. Mycologia 40: 199–231. https://doi.org/10.2307/3755085
- Chiu, W.F. (1957) Atlas of Yunnan boletes. Science Press, Beijing, 156 pp.
- Della Maggiora, M. (2015) Nomenclatural novelties. Index Fungorum 246: 1–1.
- Ellis, M.B. & Ellis, J.P. (1990) Fungi without gills (Hymenomycetes and Gasteromycetes): an identification handbook. Springer Science & Business Media, London.
- Ennamany, R., Bingen, A., Creppy, E.E., Kretz, O., Gut, J.P., Dubuisson, L., Balabaud, C., Bioulac Sage, P. & Kirn, A. (1998) Aspirin and heparin prevent hepatic blood stasis and thrombosis induced by the toxic glycoprotein Bolesatine in mice. Human & Experimental Toxicology 17 (11): 620–624. https://doi.org/10.1177/096032719801701106
- Frank, J.L. (2015) Nomenclatural novelties. Index Fungorum 248: 1–1.
- Gardes, M. & Bruns, T.D. (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
- Gouy, M., Guindon, S. & Gascuel, O. (2010) SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27 (2): 221–224. https://doi.org/10.1093/molbev/msp259
- Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98.
- Hussain, S., Al-Kharousi, M., Al-Muharabi, M.A., Al-Maqbali, D.A., Al-Shabibi, Z., Al-Balushi, A.H., Al-Yahya’ei, M.N., Al Saady, N., Abdel-Jalil, R., Velazhahan, R. & Al-Sadi, A.M. (2022) Phylogeny of Agaricus subgenus Pseudochitonia with the description of a new section and a new species from Oman. Mycological Progress 21 (8): 72. https://doi.org/10.1007/s11557-022-01819-8
- Khan, J., Kiran, M., Jabeen, S., Sher, H. & Khalid, A.N. (2017) Gymnopilus penetrans and G. swaticus sp. nov. (Agaricomycota: Hymenogastraceae); a new record and a new species from northwest Pakistan. Phytotaxa 312 (1): 60–70. https://doi.org/10.11646/phytotaxa.312.1.4
- Kretz, O., Creppy, E.E. & Dirheimer, G. (1991) Characterization of bolesatine, a toxic protein from the mushroom Boletus satanas Lenz and its effects on kidney cells. Toxicology 66 (2): 213–224. https://doi.org/10.1016/0300-483X(91)90220-U
- Mao, N., Zhao, T.Y., Zhang, Y.X., Li, T., Lv, J.C. & Fan, L. (2023) Boletaceae from Shanxi Province of northern China with descriptions of ten new species. Mycosphere 14 (1): 2013–2091. https://doi.org/10.5943/mycosphere/14/1/24
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA, pp. 1–8. https://doi.org/10.1109/GCE.2010.5676129
- Rambaut, A., Suchard, M.A., Xie, D. & Drummond, A.J. (2014) Tracer v1.6. Available from: http://beast.bio.ed.ac.uk/Tracer (accessed 22 August 2020)
- Ronquist, F. & Huelsenbeck, J.P. (2003) MrBayes3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
- Rathnayaka, A.R., Tennakoon, D.S., Jones, G.E., Wanasinghe, D.N., Bhat, D.J., Priyashantha, A.H., Stephenson, S.L., Tibpromma, S. & Karunarathna, S.C. (2025) Significance of precise documentation of hosts and geospatial data of fungal collections, with an emphasis on plant-associated fungi. New Zealand Journal of Botany 63 (2–3): 462–489.
- https://doi.org/10.1080/0028825X.2024.2381734
- Rumack, B.H. & Spoerke, D.G. (1994) Handbook of mushroom poisoning: diagnosis and treatment. CRC Press, Boca Raton.
- Sarwar, S., Siddique, Z.E.B., Bashir, A. & Khalid, A.N. (2021) Rubroboletus himalayensis—A new mushroom from Pakistan. Bangladesh Journal of Plant Taxonomy 28 (1): 17–26. https://doi.org/10.3329/bjpt.v28i1.54206
- Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246.
- https://doi.org/10.1128/jb.172.8.4238-4246.1990
- Wang, X.H., Liu, P.G. & Yu, F.Q. (2004) Color atlas of wild commercial mushrooms in Yunnan. Yunnan Science and Technology Press, Kunming, China.
- White, T.J., Bruns, T., Lee, S. & Taylor, J.W. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp. 315–322.
- https://doi.org/10.1016/b978-0-12-372180-8.50042-1
- Zhang, X., Qin, W.Q., Liang, Z.Q., Tian, R. & Zeng, N.K. (2022) Rubroboletus flammeus (Boletaceae, Boletales), a novel species unveiled from subtropical China based on morphological and phylogenetic evidences. Archives of Microbiology 204 (7): 379. https://doi.org/10.1007/s00203-022-02998-4
- Zhao, K. & Shao, H.M. (2017) A new edible bolete, Rubroboletus esculentus, from southwestern China. Phytotaxa 303 (3): 243–252. https://doi.org/10.11646/phytotaxa.303.3.4
- Zhao, K., Wu, G. & Yang, Z.L. (2014a) A new genus, Rubroboletus, to accommodate Boletus sinicus and its allies. Phytotaxa 188 (2): 61–77. https://doi.org/10.11646/phytotaxa.188.2.1
- Zhao, K., Wu, G., Feng, B. & Yang, Z.L. (2014b) Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycological Progress 13: 1127–1136. https://doi.org/10.1007/s11557-014-1001-3