Abstract
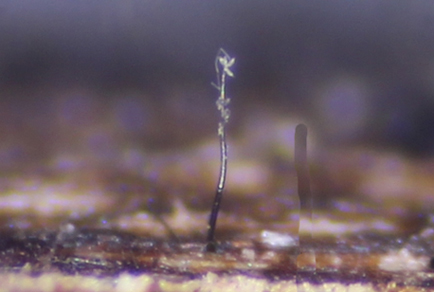
Thysanorea tongrensis sp. nov., a novel saprobic fungus colonizing decaying wood, is described and illustrated based on specimens collected in the Fodingshan National Nature Reserve, Guizhou Province, China. Phylogenetic analyses based on a combined dataset of ITS, LSU, and SSU sequences, along with detailed morphological comparisons, indicate that the new species is closely related to Thysanorea curvata. However, T. tongrensis is distinguished by its longer conidiophores (180–415 µm vs. 150–230 µm) and larger hyaline conidia (24–38 × 5–8 µm), in contrast to the smaller, pale brown conidia (16–18 × 6.5–7.5 µm) of T. curvata. Detailed morphological descriptions, illustrations, and phylogenetic analyses of the new species are presented.
References
- Arzanlou, M., Groenewald, J.Z., Gams, W., Braun, U., Shin, H.D. & Crous, P.W. (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies In Mycology 58: 57–93. https://doi.org/10.3114/sim.2007.58.03
- De Hoog, G.S., Vicente, V.A., Najafzadeh, M.J., Harrak, M.J., Badali, H. & Seyedmousavi, S. (2011) Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 27: 46–72. https://doi.org/10.3767/003158511X614258
- Diederich, P., Ertz, D. & Lawrey, J.D. (2013) Molecular data place the hyphomycetous lichenicolous genus Sclerococcum close to Dactylospora (Eurotiomycetes) and S. parmeliae in Cladophialophora (Chaetothyriales). Fungal Diversity 58: 61–72. https://doi.org/10.1007/s13225-012-0179-4
- Dong, W., Hyde, K.D. & Bhat, D.J. (2018) Introducing Aculeata aquatica gen. et sp. nov., Minimelanolocus thailandensis sp. nov. and Thysanorea aquatica sp. nov. (Herpotrichiellaceae, Chaetothyriales) from freshwater in northern Thailand. Mycological Progress 17: 617–629. https://doi.org/10.1007/s11557-018-1389-2
- Gardes, M. & Bruns, T.D. (1993) ITS primers with enhanced specificity for basidiomycetes--application to the identification of mycorrhizae and rusts. Molecular Ecology 2 (2): 113–8. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
- Geiser, D.M., Gueidan, C., Miadlikowska, J., Lutzoni, F., Kauff, F., Hofstetter, V., Fraker, E., Schoch, C.L., Tibell, L., Untereiner, W.A. & Aptroot, A. (2006) Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98 (6): 1053–64. https://doi.org/10.1080/15572536.2006.11832633
- Gueidan, C., Villaseñor, C.R., De Hoog, G.S., Gorbushina, A.A., Untereiner, W.A. & Lutzoni, F.A. (2008) Rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Studies In Mycology 61: 111–9. https://doi.org/10.3114/sim.2008.61.11
- Hall, T.A. (1999) Bioedit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nuclc Acids Symposium Series 41 (41): 95–98.
- Hernández-Restrepo, M., Giraldo, A., van Doorn, R., Wingfield, M.J., Groenewald, J.Z., Barreto, R.W., Colmán, A.A., Mansur, P.S.C. & Crous, P.W. (2020) The Genera of Fungi –G6: Arthrographis, Kramasamuha, Melnikomyces, Thysanorea and Verruconis. Fungal Systematics and Evolution 6: 1–24. https://doi.org/10.3114/fuse.2020.06.01
- Hyde, K.D., Hongsanan, S. & Jeewon, R. (2016) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 80: 1–270. https://doi.org/10.1007/s13225-016-0373-x
- Hyde, K.D., Dong, Y. & Phookamsak, R. (2019) Fungal diversity notes 1036–1150: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity 100: 5–277. https://doi.org/10.1007/s13225-020-00439-5
- James, T., Kauff, F. & Schoch, C. (2006) Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature 443: 818–822. https://doi.org/10.1038/nature05110
- Katoh, K., Rozewicki, J. & Yamada, K.D. (2019) MAffT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160‒1166. https://doi.org/10.1093/bib/bbx108
- Kirschner, R. (2016) Revision of the morphology and biogeography of Thysanorea papuana. Mycosphere 7 (6): 820–827. https://doi.org/10.5943/mycosphere/7/6/13
- Liu, X.Y., Udayanga, D. & Luo, Z.L. (2015) Backbone tree for Chaetothyriales with four new species of Minimelanolocus from aquatic habitats. Fungal Biology 119: 1046–1062. https://doi.org/10.1016/j.funbio.2015.08.005
- Liu, L., Zhang, Q., Ren, Y., Yang, Q., Li, Q., Lin, C., Wijayawardene, N., Cheewangkoon, R., Liu, H. & Samarakoon, M.C. (2024) Craspedodidymum hunanense sp. nov. (Chaetosphaeriaceae, Chaetosphaeriales) and a new record of Aquadictyospora clematidis (Dictyosporiaceae, Pleosporales), from Hunan Province, China. Phytotaxa 675 (1): 43–58. https://doi.org/10.11646/phytotaxa.675.1.4
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: 2010 Gateway Computing Environments Workshop (GCE). IEEE, pp. 1–8. https://doi.org/10.1109/GCE.2010.5676129
- Rambaut, A. (2018) FigTree: tree figure drawing tool (Version 1.4.4).
- Réblová, M., Untereiner, W.A. & Réblová, K. (2013) Novel evolutionary lineages revealed in the Chaetothyriales (Fungi) based on multigene phylogenetic analyses and comparison of ITS secondary structure. Plos one 8 (5): e63547. https://doi.org/10.1371/journal.pone.0063547
- Ronquist, F., Teslenko, M., van der Mark, P & Ayres, D.L. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Saeed, A. (2011) Chinese western region’s development programme. Strategic Studies 31: 89–107.
- Schoch, C.L., Robbertse, B., Robert, V., Vu, D., Cardinali, G., Irinyi, L., Meyer, W. & Nilsson, R.H. (2014) Finding needles in haystacks: linking scientific names, reference specimens and molecular data for Fungi. The Journal of Biological Databases and Curation 2014: bau061. https://doi.org/10.1093/database/bau061
- Shen, H.W., Bao, D.F., Bhat, D.J., Su, H.Y. & Luo, Z.L. (2022) Lignicolous freshwater fungi in Yunnan province, China: An Overview. Mycology 13 (2): 119–32. https://doi.org/10.1080/21501203.2022.2058638
- Stamatakis, A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30 (9): 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Tian, Q., Doilom, M., Luo, Z.L., Chomnunti, P., Bhat, D.J., Xu, J.C. & Hyde, K.D. (2016) Introducing Melanoctona tectonaegen. et sp. nov. and Minimelanolocus yunnanensis sp. nov. (Herpotrichiellaceae, Chaetothyriales), Cryptogamie, Mycologia 37 (4): 477–492. https://doi.org/10.7872/crym/v37.iss4.2016.477
- Untereiner, W.A. & Naveau, F.A. (1999) Molecular systematics of the Herpotrichiellaceae with an assessment of the phylogenetic positions of Exophiala dermatitidis and Phialophora americana. Mycologia 91: 67–83. https://doi.org/10.1080/00275514.1999.12060994
- Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
- Vu, D., Groenewald, M., de Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., Al-Hatmi, A., Groenewald, J.Z., Groenewald, G., Cardinali, J., Houbraken, T., Boekhout, P.W., Crous, V., Robert, G.J. & Verkley, M. (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation, Studies in Mycology 92: 135–154. https://doi.org/10.1016/j.simyco.2018.05.001
- Wang, G.N., Yu, X.D. & Dong, W. (2019) Freshwater hyphomycetes in Eurotiomycetes: a new species of Minimelanolocus and a new collection of Thysanorea papuana (Herpotrichiellaceae). Mycol Progress 18: 511–522. https://doi.org/10.1007/s11557-019-01473-7
- Wang, S.J., Li, R.L., Sun, C.X., Zhang, D. & Li, F.Q. (2004) How types of carbonate rock assemblages constrain the distribution of karst rocky decertified land in Guizhou Province, PR China: phenomena and mechanisms. Land Degradation & Development 15 (2): 123–131. https://doi.org/10.1002/ldr.591
- Wan, Y.L., Bao, D.F., Luo, Z.L. & Bhat, D.J. (2021) Two new species of Minimelanolocus (Herpotrichiellaceae, Chaetothyriales) from submerged wood in Yunnan, China. Phytotaxa 480 (1): 45–56. https://doi.org/10.11646/phytotaxa.480.1.4
- White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: A Guide to Methods and Applications 18: pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- Wijayawardene, N.N., Dissanayake, L.S., Dai, D.Q., Li, Q.R., Xiao, Y., Wen, T.C., Karunarathna, S.C., Wu, H.X., Zhang, H., Tibpromma, S. & Kang, J.C. (2021) Yunnan-Guizhou plateau: a mycological hotspot. Phytotaxa 523 (1): 1–31. https://doi.org/10.11646/phytotaxa.523.1.1
- Yang, J., Liu, L.L., Jones, E., Hyde, K.D., Liu, Z.Y. & Bao, D.F. (2023) Freshwater fungi from karst landscapes in China and Thailand. Fungal Diversity 119: 1–212. https://doi.org/10.1007/s13225-023-00514-7