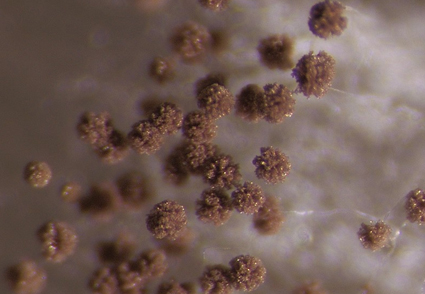
Abstract
This study introduces two new black Aspergillus species, A. dhakephalkarii and A. patriciawiltshireae, in sect. Nigri ser. Japonici. Aspergillus dhakephalkarii and A. patriciawiltshireae were isolated from soils from the Western Ghats of India. The taxonomy of the new species was determined through a polyphasic approach that included phenotypic characteristics and phylogenetic analyses of the ribosomal internal transcribed spacer (ITS) region, and partial beta-tubulin (BenA), calmodulin (CaM) and RNA polymerase II second largest subunit (RPB2) genes applying Genealogical Concordance Phylogenetic Species Recognition (GCPSR). The main characteristics of A. dhakephalkarii include: fast-growing colonies with the production of pale to dark brown to brown conidia en masse; yellowish white to yellowish orange sclerotia; uniseriate conidiophores splitting into two to three columns; and smooth-walled ellipsoidal conidia. The conidial features of the new species differ from those of closely related species in the series, which typically have spherical conidia and echinulate walls. A. patriciawiltshireae is characterised by fast growing colonies with abundant sclerotia production on Czapek Yeast Autolysate Agar (CYA) and Malt Extract Agar (MEA) and modest sporulation. Aspergillus patriciawiltshireae has echinulate conidia, and uniseriate conidiophores, splitting into more than five columns. Phylogenetically, A. dhakephalkarii is sister to A. saccharolyticus, and A. patriciawiltshireae to A. indologenus, A. japonicus, and A. uvarum. This study also reports the first records of A. aculeatinus and A. brunneoviolaceous from India.
References
- Abadi, S., Azouri, D., Pupko, T. & Mayrose, I. (2019) Model selection may not be a mandatory step for phylogeny reconstruction. Nature Communications 10: 934. https://doi.org/10.1038/s41467-019-08822-w
- Agnihothrudu, V. (1957) Fungi isolated from rhizosphere. II. Starkeomyces, a new genus of the Tuberculariaceae. Journal of the Indian Botanical Society 35 (1): 38–42.
- Aljanabi, S.M. & Martinez, I. (1997) Universal and rapid salt-extraction of high-quality genomic DNA for PCR-based techniques. Nucleic Acids Research 25 (22): 4692–4703. https://doi.org/10.1093/nar/25.22.4692
- Al-Musallam, A. (1980) Revision of the black Aspergillus species. PhD Thesis, Utecht University, Centraalbureau voor Schimmelcultures, Baarn.
- Bennett, S.E. (2007) An overview of the genus Aspergillus. In: Goldman, G.H. & Osmani, S.A. (Eds.) The Aspergilli. CRC Press, pp. 23–34.
- Bian, C., Kusuya, Y., Sklenář, F., D'hooge, E., Yaguchi, T., Ban, S., Visagie, C.M., Houbraken, J., Takahashi, H. & Hubka, V. (2022) Reducing the number of accepted species in Aspergillus series Nigri. Studies in Mycology 102 (1): 95–132. https://doi.org/10.3114/sim.2022.102.03
- Bilgrami, K.S., Jamaluddin, S. & Rizwi, M.A. (1991) Fungi of India. List and References. Edn 2, revised, enlarged and brought up to date. Today and Tomorrow’s Printers & Publishers. New Delhi, India. [vii] + 798 pp.
- Chauhan, S.K., Tyagi, V.K. & Nagar, M.L. (1980) Mycoflora of soil around pneumatophores of Soneratia acida L. in Andaman Islands. Journal of the Indian Botanical Society 59 (4): 281–285.
- Chen, A.J., Hubka, V., Frisvad, J.C., Visagie, C.M., Houbraken, J., Meijer, M., Varga, J., Demirel, R.A., Jurjević, Ž., Kubátová, A. & Sklenář, F. (2017) Polyphasic taxonomy of Aspergillus section Aspergillus (formerly Eurotium), and its occurrence in indoor environments and food. Studies in Mycology 88: 37–135.
- Crous, P.W., Wingfield, M.J., Burgess, T.I., Hardy, G.E.S.J., Gené, J., Guarro, J., Baseia, I.G., García, D., Gusmão, L.F.P., Souza-Motta, C.M., Thangavel, R., Adamčík, S., Barili, A., Barnes, C.W., Bezerra, J.D.P., Bordallo, J.J., Cano-Lira, J.F., de Oliveira, R.J.V., Ercole, E., Hubka, V., Iturrieta-González, I., Kubátová, A., Martín, M.P., Moreau, P.A., Morte, A., Ordoñez, M.E., Rodríguez, A., Stchigel, A.M., Vizzini, A., Abdol- lahzadeh, J., Abreu, V.P., Adamčíková, K., Albuquerque, G.M.R., Alexandrova, A.V., Álvarez Duarte, E., Armstrong-Cho, C., Banniza, S., Barbosa, R.N., Bellanger, J.M., Bezerra, J.L., Cabral, T.S., Caboň, M., Caicedo, E., Cantillo, T., Carnegie, A.J., Carmo, L.T., Castañeda-Ruiz, R.F., Clement, C.R., Čmoková, A., Conceição, L.B., Cruz, R.H.S.F., Damm, U., da Silva, B.D.B., da Silva, G.A., da Silva, R.M.F., de Santiago, A.L.C.M., de Oliveira, L.F., de Souza, C.A.F., Déniel, F., Dima, B., Dong, G., Edwards, J., Félix, C.R., Fournier, J., Gibertoni, T.B., Hosaka, K., Iturriaga, T., Jadan, M., Jany, J.L., Jurjević, Ž., Kolařík, M., Kušan, I., Landell, M.F., Leite Cordeiro, T.R., Lima, D.X., Loizides, M., Luo, S., Machado, A.R., Madrid, H., Magalhães, O.M.C., Marinho, P., Matočec, N., Mešić, A., Miller, A.N., Morozova, O.V., Neves, R.P., Nonaka, K., Nováková, A., Oberlies, N.H., Oliveira-Filho, J.R.C., Oliveira, T.G.L., Papp, V., Pereira, O.L., Perrone, G., Peterson, S.W., Pham, T.H.G. & Raja, H.A. (2018) Fungal Planet description sheets: 716–784. Persoonia: Molecular Phylogeny and Evolution of Fungi 40: 239–392. https://doi.org/10.3767/persoonia.2018.40.10
- Crous, P.W., Wingfield, M.J., Chooi, Y.H., Gilchrist, C.L.M., Lacey, E., Pitt, J.I., Roets, F., Swart, W.J., Cano-Lira, J.F., Valenzuela-Lopez, N., Hubka, V., Shivas, R.G., Stchigel, A.M., Holdom, D.G., Jurjević, Ž., Kachalkin, A.V., Lebel, T., Lock, C., Martín, M.P., Tan, Y.P., Tomashevskaya, M.A., Vitelli, J.S., Baseia, I.G., Bhatt, V.K., Brandrud, T.E., De Souza, J.T., Dima, B., Lacey, H.J., Lombard, L., Johnston, P.R., Morte, A., Papp, V., Rodríguez, A., Rodríguez-Andrade, E., Semwal, K.C., Tegart, L., Abad, Z.G., Akulov, A., Alvarado, P., Alves, A., Andrade, J.P., Arenas, F., Asenjo, C., Ballarà, J., Barrett, M.D., Berná, L.M., Berraf-Tebbal, A., Virginia Bianchinotti, M., Bransgrove, K., Burgess.T.I., Carmo, F.S., Chávez, R., Čmoková, A., Dearnaley, J.D.W., André Luiz, A.L.C.M., Freitas-Neto, J.F., Denman, S., Douglas, B., Dovana, F., Eichmeier, A., Esteve-Raventós, F., Farid, A., Fedosova, A.G., Ferisin, G., Ferreira, R.J., Ferrer, A., Figueiredo, C.N., Figueiredo, Y.F., Reinoso-Fuentealba, C.G., Garrido-Benavent, I., Cañete-Gibas, C.F., Gil-Durán, C., Glusha-kova, A.M., Gonçalves, M.F.M., González, M., Gorczak, M., Gorton, C., Guard, F.E., Guarnizo, A.L., Guarro, J., Gutiérrez, M., Hamal, P., Hien, L.T., Hocking, A.D., Houbraken, J., Hunter, G.C., Inácio, C.A., Jourdan, M., Kapitonov, V.I., Kelly, L., Khanh, T.N., Kisło, K., Kiss, L., Kiyashko, A., Kolařík, M., Kruse, J., Kubátová, A., Kučera, V., Kučerová, I., Kušan, I., Lee, H.B., Levicán, G., Lewis, A., Van, L.N., Liimatainen, K., Lim, H.J., Lyons, M.N., Maciá-Vicente, J.G., Magaña-Dueñas, V., Mahiques, R., Malysheva, E.F., Marbach, P.A.S., Marinho, P., Matočec, N., McTaggart, A.R., Mešić, A., Morin, L., Muñoz-Mohedano, J.M., Navarro-Ródenas, A., Nicolli, C.P., de Oliveira, R.L., Otsing, E., Ovrebo, C.L., Pankratov, T.A., Paños, A., Paz-Conde, A., Pérez-Sierra, A., Phosri, C., Pintos, Á., Pošta, A., Prencipe, S., Rubio, E., Saitta, A., Sales, L.S., Sanhueza, L., Shuttleworth, L.A., Smith, J., Smith, M.E., Spadaro, D., Spetik, M., Sochor, M., Sochorová, Z., Sousa, J.O., Suwan-nasai, N., Tedersoo, L., Thanh, H.M., Thao, L.D., Tkalčec, Z., Vaghefi, N., Venzhik, A.S., Verbeken, A., Vizzini, A., Voyron, S., Wainhouse, M., Whalley, A.J.S., Wrzosek, M., Zapata, M., Zeil-Rolfe, I. & Groenewald, J.Z. (2020) Fungal planet description sheets: 1042–1111. Persoonia: Molecular Phylogeny and Evolution of Fungi 44: 301–459. https://doi.org/10.3767/persoonia.2020.44.11
- Doilom, M., Guo, J.W., Phookamsak, R., Mortimer, P.E., Karunarathna, S.C., Dong, W., Liao, C.F., Yan, K., Pem, D., Suwannarach, N. & Promputtha, I. (2020) Screening of phosphate-solubilizing fungi from air and soil in Yunnan, China: Four novel species in Aspergillus, Gongronella, Penicillium, and Talaromyces. Frontiers in microbiology 11: 585215. https://doi.org/10.3389/fmicb.2020.585215
- Domsch, K.H., Gams, W. & Anderson, T.H. (1980) Compendium of Soil Fungi. London: Academic Press. 859 pp.
- Fungaro, M.H.P., Ferranti, L.S., Massi, F.P., da Silva, J.J., Sartori, D., Taniwaki, M.H., Frisvad, J.C. & Lamanaka, B.T. (2017) Aspergillus labruscus sp. nov., a new species of Aspergillus section Nigri discovered in Brazil. Scientific Reports 7: 6203.
- Geiser, D.M., Samson, R.A., Varga, J., Rokas, A. & Witiak, S.M. (2008) A review of molecular phylogenetics in Aspergillus and prospects for a robust genus-wide phylogeny. In: Varga, K. & Samson, R.A. (eds.) Aspergillus in the genomic era. Academic Publishers, Wageningen, pp. 17–32.
- Glass, N.L. & Donaldson, G.C. (1995) Development of premier sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
- Glässnerová, K., Sklenář, F., Jurjević, Ž., Houbraken, J., Yaguchi, T., Visagie, C.M., Gené, J., Siqueira, J.P., Kubátová, A., Kolařík, M. & Hubka, V. (2022) A monograph of Aspergillus section Candidi. Studies in Mycology 102 (1): 1–51.
- Gokulapalan, C. & Nair, M.C. (1994) Phylloplane fungi of rice from Kerala. Indian Phytopathology 47 (4): 440.
- Hawksworth, D.L. (1974) Mycologist's Handbook: An introduction to the principles of taxonomy and nomenclature in the fungi and lichens. Commonwealth Mycological Institute, Kew, United Kingdom.
- Hong, S.B., Cho, H.S., Shin, H.D., Frisvad, J.C. & Samson, R.A. (2006) Novel Neosartorya species isolated from soil in Korea. International Journal of Systematic and Evolutionary Microbiology 56 (2): 477– 486. https://doi.org/10.1099/ijs.0.63980-0
- Houbraken, J., de Vries, R.P. & Samson, R.A. (2014) Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Advances in applied microbiology 86: 199–249. https://doi.org/10.1016/B978-0-12-800262-9.00004-4
- Houbraken, J., Kocsubé, S., Visagie, C.M., Yilmaz, N., Wang, X.C., Meijer, M., Kraak, B., Hubka, V., Bensch, K., Samson, R.A. & Frisvad, J.C. (2020) Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Studies in Mycology 95: 51–69. https://doi.org/10.1016/j.simyco.2020.05.002
- Houbraken, J. & Samson, R.A. (2011) Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology 70: 1–51.
- Jurjević, Ž., Peterson, S.W., Stea, G., Solfrizzo, M., Varga, J., Hubka, V. & Perrone, G. (2012) Two novel species of Aspergillus section Nigri from indoor air. IMA fungus 3 (2): 159–73. https://doi.org/10.5598/imafungus.2012.03.02.08
- Kamal, K. & Bhargava, K.S. (1972) Studies on soil fungi from teak forests of Gorakhpur - II. A contribution to Indian aspergilli. Mycopathologia et Mycologia Applicata 47 (12): 59–72.
- Kamal, K. & Singh, C.S. (1970) On rhizosphere fungal flora of some ferns from Darjeeling (India). Annales de l’Institute Pasteur 119: 360–368
- Kamal, K. & Singh, N.P. (1974) On microfungi from root region of ten sugarcane varieties. Indian Phytopathology 27 (3): 347–354
- Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30 (4): 772–780.
- Khuna, S., Kumla, J., Srinuanpan, S., Lumyong, S. & Suwannarach, N. (2023) Multifarious Characterization and Efficacy of Three Phosphate-Solubilizing Aspergillus Species as Biostimulants in Improving Root Induction of Cassava and Sugarcane Stem Cuttings. Plants 12 (20): 3630. https://doi.org/10.3390/plants12203630
- Khuna, S., Suwannarach, N., Kumla, J., Frisvad, J.C., Matsui, K., Nuangmek, W. & Lumyong, S. (2021) Growth Enhancement of Arabidopsis (Arabidopsis thaliana) and Onion (Allium cepa) With Inoculation of Three Newly Identified Mineral-Solubilizing Fungi in the Genus Aspergillus Section Nigri. Frontiers in Microbiology 12: 705896. https://doi.org/10.3389/fmicb.2021.705896
- Klich, M.A. (2002) Biogeography of Aspergillus species in soil and litter. Mycologia 94 (1): 21–27. https://doi.org/10.1080/15572536.2003.11833245
- Klich, M.A. (2002) Identification of common Aspergillus species. CBS, Utrecht.
- Kozakiewicz, Z. (1989) Aspergillus species on stored products. CAB International, Wallingford.
- Liu, Y.J., Whelen, S. & Hall, B.D. (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular biology and evolution 16: 1799–1808. https://doi.org/10.1093/oxfordjournals.molbev.a026092
- Maia, T.F., Simões-Araújo, J.L., Inácio, C.A. & Fraga, M.E. (2015) Characterization of Aspergillus section Nigri isolates from leaf litter and soil in the Atlantic Forest of Brazil. African Journal of Microbiology Research 9 (5): 301–306. https://doi.org/10.5897/AJMR2014.7130
- Mehrotra, B.S. & Basu, M. (1976) Fungi associated with stored wheat and its milling fractions in India. Indian Journal of Mycology and Plant Pathology 6 (1): 43–50.
- Minh, B.Q., Nguyen, M.A.T. & Von Haeseler, A. (2013) Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30: 1188–1195.
- Mittermeier, R.A., Gils, P.R., Hofmann, M., Pilgrim, J., Brooks, T., Mitermeier, C.G., Lamoreaux, J. & da Fonseca, G.A.B. (2004) Hotspots Revisited. Earth’s Biologically Richest and Most Endangered Terrestrial Ecosystems. Conservation International, CEMEX, Mexico City, Mexico, 392 pp.
- Nguyen, L.T., Schmidt, H.A., von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32 (1): 268–274. https://doi.org/10.1093/molbev/msu300
- Peterson, S.W. (2008) Phylogenetic analyses of Aspergillus species using DNA sequences from four loci. Mycologia 100: 205–226.
- Peterson, S.W., Varga, J., Frisvad, J.C. & Samson, R.A. (2008) Phylogeny and subgeneric taxonomy of Aspergillus. In: Varga, J. & Samson, R.A. (eds.) Aspergillus in the genomic era. Wageningen Academic Publishers, Wageningen, pp. 33–56. https://doi.org/10.3920/9789086866359_003
- Pitt, J.I. & Hocking, A.D. (2009) Fungi and food spoilage. 4th edition. Springer, Switzerland.
- Pramer, D. & Schmidt, E.L. (1964) Experimental soil microbiology. Soil Science 98 (3): 211.
- Raper, K.B. & Fennel, D.I. (1965) The Genus Aspergillus. The Williams and Wilkins Company. Baltimore.
- Samson, R.A., Hoekstra, E.S. & Frisvad, J.C. (2004) Introduction to Food and Airborne Fungi. 7th edn. Centraalbureau voor Schimmelcultures, Utrecht.
- Samson, R.A., Noonim, P., Meijer, M., Houbraken, J.A., Frisvad, J.C. & Varga, J. (2007) Diagnostic tools to identify black aspergilli. Studies in Mycology 59 (1): 129–145. https://doi.org/10.3114/sim.2007.59.13
- Samson, R.A., Visagie, C.M., Houbraken, J., Hong, S.B., Hubka, V., Klaassen, C.H., Perrone, G., Seifert, K.A., Susca, A., Tanney, J.B. & Varga, J. (2014) Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology 78 (1): 141–173. https://doi.org/10.1016/j.simyco.2014.07.004
- Silva, D.M., Batista, L.R., Rezende, E.F., Fungaro, M.H., Sartori, D. & Alves, E. (2011) Identification of fungi of the genus Aspergillus section Nigri using polyphasic taxonomy. Brazilian Journal of Microbiology 42: 761–773. https://doi.org/10.1590/S1517-83822011000200044
- Sklenář, F., Glässnerová, K., Jurjević, Ž., Houbraken, J., Samson, R.A., Visagie, C.M., Yilmaz, N., Gené, J., Cano, J., Chen, A.J. & Nováková, A. (2022) Taxonomy of Aspergillus series Versicolores: species reduction and lessons learned about intraspecific variability. Studies in Mycology (1): 53–93.
- Sklenář, F., Jurjević, Ž., Zalar, P., Frisvad, J.C., Visagie, C.M., Kolařík, M., Houbraken, J., Chen, A.J., Yilmaz, N., Seifert, K.A. & Coton, M. (2017) Phylogeny of xerophilic aspergilli (subgenus Aspergillus) and taxonomic revision of section Restricti. Studies in mycology 88: 161–236.
- Sørensen, A., Lübeck, P.S., Lübeck, M., Nielsen, K.F., Ahring, B.K., Teller, P.J. & Frisvad, J.C. (2011) Aspergillus saccharolyticus sp. nov., a black Aspergillus species isolated in Denmark. International journal of systematic and evolutionary microbiology 61 (12): 3077–3083. https://doi.org/10.1099/ijs.0.029884-0
- Varga, J., Frisvad, J.C., Kocsubé, S., Brankovics, B., Tóth, B., Szigeti, G. & Samson, R. (2011) New and revisited species in Aspergillus section Nigri. Studies in Mycology 69 (1): 1–7. https://doi.org/10.3114/sim.2011.69.01
- Visagie, C.M. & Houbraken, J. (2020) Updating the taxonomy of Aspergillus in South Africa. Studies in Mycology 95 (1): 253–292. https://doi.org/10.1016/j.simyco.2020.02.003
- Visagie, C.M., Yilmaz, N., Kocsubé, S., Frisvad, J.C., Hubka, V., Samson, R.A. & Houbraken, J. (2024) A review of recently introduced Aspergillus, Penicillium, Talaromyces and other Eurotiales species. Studies in Mycology 107: 1–66. https://doi.org/10.3114/sim.2024.107.01
- Wang, X., Jarmusch, S.A., Frisvad, J.C. & Larsen, T.O. (2023) Current status of secondary metabolite pathways linked to their related biosynthetic gene clusters in Aspergillus section Nigri. Natural Product Reports 40 (2): 237–274. https://doi.org/10.1039/D1NP00074H
- White, T.J., Bruns, T., Lee, J. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- Zhou, Y.B., Rezaei-Matehkolaei, A., Meijer, M., Kraak, B., Gerrits van den Ende, B., Hagen, F., Afzalzadeh, S., Kiasat, N., Takesh, A., Hoseinnejad, A. & Houbraken, J. (2024) Aspergillus hubkae, a Novel Species Isolated from a Patient with Probable Invasive Pulmonary Aspergillosis. Mycopathologia 189 (3): 44.