Abstract
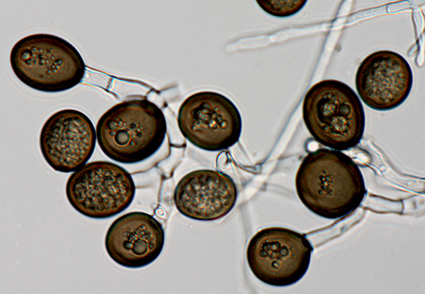
A routine survey in a natural forest located in the Gangwon Province of Korea was undertaken in 2022 to establish an inventory of potentially pathogenic fungi that might affect tree health in the country. An ophiostomatoid fungus was consistently isolated from naturally occurring wounds on Prunus serrulata var. pubescens. Isolates were subjected to morphological and DNA sequence comparisons. The results showed that the fungus resided in a distinct taxonomic lineage, and the novel species is described here as Chalaropsis pruni sp. nov.
References
- de Beer, Z.W., Duong, T., Barnes, I., Wingfield, B.D. & Wingfield, M.J. (2014) Redefining Ceratocystis and allied genera. Studies in Mycology 79: 187–219. https://doi.org/10.1016/j.simyco.2014.10.001
- de Hoog, G. & Scheffer, R. (1984) Ceratocystis versus Ophiostoma: a reappraisal. Mycologia 76: 292–299. https://doi.org/10.1080/00275514.1984.12023838
- Gardes, M. & Bruns, T.D. (1993) ITS primers with enhanced specificity for basidiomycetes‐application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
- Heath, R.N., Wingfield, M.J., Van Wyk, M. & Roux, J. (2009) Insect associates of Ceratocystis albifundus and patterns of association in a native savanna ecosystem in South Africa. Environmental Entomology 38: 356–364. https://doi.org/10.1603/022.038.0207
- Katoh, K., Misawa, K. & Kuma, K. (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. https://doi.org/10.1093/nar/gkf436
- Kile, G.A. (1993) Plant diseases caused by species of Ceratocystis sensu stricto and Chalara. In: Wingfield, M.J., Seifert, K.A. & Webber, J. (Eds.) Ceratocystis and ophiostoma: taxonomy, ecology and pathogenicity. APS Press, St. Paul, Minnesota, pp. 173–183.
- Kumar, S., Stecher, G. & Tamura, K. (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. https://doi.org/10.1093/molbev/msw054
- Li, X., Li, J., Bai, Y.H., Xu, K.C., Zhang, R.Q. & Huang, Q. (2021) First report of wilt of rubber tree caused by Chalaropsis thielavioides in China. Plant Disease 105: 1221. https://doi.org/10.1094/PDIS-09-20-2066-PDN
- Maharachchikumbura, S.S.N., Chen, Y., Ariyawansa, H.A., Hyde, K.D., Haelewaters, D., Perera, R.H., Samarakoon, M.C., Wanasinghe, D.N., Bustamante, D.E., Liu, J., Lawrence, D.P., Cheewangkoon, R. & Stadler, M. (2021) Integrative approaches for species delimitation in Ascomycota. Fungal Diversity 109: 155–179. https://doi.org/10.1007/s13225-021-00486-6
- Moller, W.J. & DeVay, J.E. (1968) Insect transmission of Ceratocystis fìmbriata in deciduous fruit orchards. Phytopathology 58: 1499–1508.
- Nag Raj, T.R. & Kendrick, W.B. (1975) A monograph of Chalara and allied genera. Wilfrid Laurier University Press, Waterloo, Ontario, USA.
- Peyronel, B. (1916) Una nuova malattia del lupino prodotta da Chalaropsis thielavioides Peyr. Le Stazioni Sperimentali Agrarie Italiane 49: 583–596.
- Roux, J. & Wingfield, M.J. (2009) Ceratocystis species: emerging pathogens of non-native plantation Eucalyptus and Acacia species. Southern Forests: a Journal of Forest Science 71: 115–120. https://doi.org/10.2989/SF.2009.71.2.5.820
- Roux, J. & Wingfield, M.J. (2013) Ceratocystis species on the African continent, with particular reference to C. albifundus, and African species in the C. fimbriata sensu lato species complex. In: Seifert, K.A., de Beer, Z.W. & Wingfield, M.J. (Eds.) The ophiostomatoid fungi: expanding frontiers. CBS biodiversity series, vol. 12. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands, 131–138 pp.
- Seifert, K.A., Wingfield, M.J. & Kendrick, W.B. (1993) A nomenclator for described species of Ceratocystis, Ophiostoma, Ceratocystiopsis, Ceratostomella and Sphaeronaemella. In: Wingfield, M.J., Seifert, K.A. & Webber, J. (Eds.) Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. APS Press, St. Paul, Minnesota, pp. 269–287.
- Seifert, K.A., de Beer, Z.W. & Wingfield, M.J. (2013) The ophiostomatoid fungi: expanding frontiers. CBS biodiversity series: vol. 12. CBS-KNAW Biodi- versity Centre, Utrecht, The Netherlands.
- Stamatakis, A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. https://doi.org/10.1093/bioinformatics/btl446
- Stamatakis, A., Hoover, P. & Rougemont, J. (2008) A rapid bootstrap algorithm for the RAxML web servers. Systematic biology 57: 758–771. https://doi.org/10.1080/10635150802429642
- Stielow, J.B., Lévesque, C.A., Seifert, K.A., Meyer, W., Irinyi, L., Smits, D., Renfurm, R., Verkley, G.J.M., Groenewald, M., Chaduli, D., Lomascolo, A., Welti, S., Lesage-Meessen, L., Favel, A., Al-Hatmi, A.M.S., Damm, U., Yilmaz, N., Houbraken, J., Lombard, L., Quaedvlieg, W., Binder, M., Vaas, L.A.I., Vu, D., Yurkov, A., Begerow, D., Roehl, O., Guerreiro, M., Fonseca, A., Samerpitak, K., van Diepeningen, A.D., Dolatabadi, S., Moreno, L.F., Casaregola, S., Mallet, S., Jacques, N., Roscini, L., Egidi, E., Bizet, C., Garcia-Hermoso, D., Martín, M.P., Deng, S., Groenewald, J.Z., Boekhout, T., de Beer, Z.W., Barnes, I., Duong, T.A., Wingfield, M.J., de Hoog, G.S., Crous, P.W., Lewis, C.T., Hambleton, S., Moussa, T.A.A., Al-Zahrani, H.S., Almaghrabi, O.A., Louis-Seize, G., Assabgui, R., McCormick, W., Omer, G., Dukik, K., Cardinali, G., Eberhardt, U., de Vries, M. & Robert, V. (2015) One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35: 242‐263. https://doi.org/10.3767/003158515X689135
- Upadhyay, H.P. (1981) A monograph of Ceratocystis and Ceratocystiopsis. University of Georgia Press, Athens, GA, USA.
- Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of bacteriology 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
- White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) PCR protocols: a guide to methods and application. Academic Press, San Diego, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- Wijayawardene, N.N., Hyde, K.D., Dai, D.Q., Sanchez-Garcia, M., Goto, B.T., Saxena, R.K., Erdogdu, M., Selçuk, F., Rajeshkumar, K.C., Aptroot, A., Błaszkowski, J., Boonyuen, N., da Silva, G.A., de Souza, F.A., Dong, W., Ertz, D., Haelewaters, D., Jones, E.B.G., Karunarathna, S.C., Kirk, P.M., Kukwa, M., Kumla, J., Leontyev, D.V., Lumbsch, H.T., Maharachchikumbura, S.S.N., Marguno, F., Martínez-Rodríguez, P., Mešić, A., Monteiro, J.S., Oehl, F., Pawłowska, J., Pem, D., Pfliegler, W.P., Phillips, A.J.L., Pošta, A., He, M.Q., Li, J.X., Raza, M., Sruthi, O.P., Suetrong, S., Suwannarach, N., Tedersoo, L., Thiyagaraja, V., Tibpromma, S., Tkalčec, Z., Tokarev, Y.S., Wanasinghe, D.N., Wijesundara, D.S.A., Wimalaseana, S.D.M.K., Madrid, H., Zhang, G.Q., Gao, Y., Sánchez-Castro., Tang, L.Z., Stadler, M., Yurkov, A. & Thines, M. ( 2022) Outline of Fungi and fungus-like taxa—2021. Mycosphere 13: 53–453 https://doi.org/10.5943/mycosphere/13/1/2
- Xu, K., Li, J., Yang, X., Zhang, R., Li, X., Xie, M. & Huang, Q. (2020) Postharvest rot on carrot caused by Ceratocystis fimbriata and Chalaropsis thielavioides (≡ Thielaviopsis thielavioides) in China. Journal of General Plant Pathology 86: 322–325. https://doi.org/10.1007/s10327-020-00919-1