Abstract
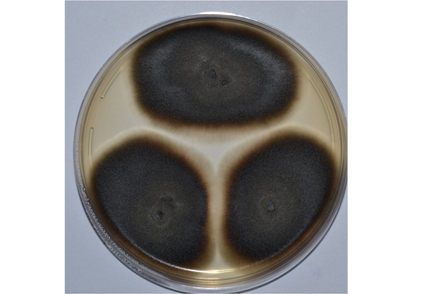
A novel species in genus Cadophora is described as Cadophora arctica. The new species was isolated from, Franz Josef Land, Russia and is represented by ex-type strain VKM F-5011. To characterize the species, we used a polyphasic taxonomic approach. A description based on morphological characters is given and it is shown that the new species is morphologically different from closely related species (to C. caespitosa, C. fastigiata, C. melinii and C. rotunda). Partial sequences of internal transcribed spacer rDNA region (ITS1-5.8S-ITS2), D1/D2 region of 28S rDNA (LSU), the loci encoding β-tubulin (BenA) and partial translation elongation factor 1-α gene (TEF1) were analyzed as well. Sequences data, macro- and micromorphological characteristics distinguish C. arctica from all known species in genus Cadophora. C. arctica, isolated from Arctic drift wood, is able to grow at 2°C and is considered a psychrotolerant species.
References
- Aleksandrova, V.D. (1977) Geobotanical zoning of the Arctic and Antarctic. Nauka, Leningrad, 189 pp.
- Arenz, B.E. & Blanchette, R.A. (2009) Investigations of fungal diversity in wooden structures and soils at historic sites on the Antarctic Peninsula. Canadian Journal of Microbiology 55: 46–56. https://doi.org/10.1139/W08-120
- Barr, S. (1995) Franz josef land. Norsk Polarinstitutt, Oslo, 175 pp.
- Bien, S. & Damm, U. (2020) Arboricolonus simplex gen. et sp. nov. and novelties in Cadophora, Minutiella and Proliferodiscus from Prunus wood in Germany. MycoKeys 63: 119–161. https://doi.org/10.3897/mycokeys.63.46836
- Blanchette, R.A, Held, B.W., Jurgens, J., Stear, A. & Dupont, C. (2021) Fungi attacking historic wood of Fort Conger and the Peary Huts in the High Arctic. PLoS ONE 16: e0246049. https://doi.org/10.1371/journal.pone.0246049
- Blanchette, R.A., Held, B.W., Arenz, B.E., Jurgens, J.A., Baltes, N.J., Duncan, S.M. & Farrell, R.L. (2010) An Antarctic hot spot for fungi at Shackleton’s historic hut on Cape Royds. Microbial Ecology 60: 29–38. https://doi.org/10.1007/s00248-010-9664-z
- Blanchette, R.A., Held, B.W., Hellmann, L., Millman, L. & Buntgen, U. (2016) Arctic driftwood reveals unexpectedly rich fungal diversity. Fungal Ecology 23: 58–65. https://doi.org/10.1016/j.funeco.2016.06.001
- Blanchette, R.A., Held, B.W., Jurgens, J.A. McNew, D.L., Harrington, T.C., Duncan, S.M. & Farrell, R.L. (2004) Wood destroying soft rot fungi in the historic expedition huts of Antarctica. Applied and Environmental Microbiology 70: 1328–1335. https://doi.org/10.1128/AEM.70.3.1328-1335.2004
- Bubnova, E.N. & Nikitin, D.A. (2017) Fungi in bottom sediments of the Barents and Kara seas. Russian Journal of Marine Biology 43: 400–406.
- https://doi.org/10.1134/S1063074017050029
- Carbone, I. & Kohn, L.M. (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. https://doi.org/10.2307/3761358
- Chen., Q., Bakhshi, M., Balci, Y., Broders, K.D., Cheewangkoon, R., Chen, S.F., Fan, X.L., Gramaje, D., Halleen, F., Horta Jung, M., Jiang, N., Jung, T., Májek, T., Marincowitz, S., Milenković, T., Mostert, L., Nakashima, C., Nurul Faziha, I., Pan, M., Raza, M., Scanu, B., Spies, C.F.J., Suhaizan, L., Suzuki, H., Tian, C.M., Tomšovský, M., Úrbez-Torres, J.R., Wang, W., Wingfield, B.D., Wingfield, M.J., Yang, Q., Yang, X., Zare, R., Zhao, P., Groenewald, J.Z., Cai, L. & Crous, P.W. (2022) Genera of phytopathogenic fungi: GOPHY 4. Studies in Mycology 101: 417–564. https://doi.org/10.3114/sim.2022.101.06
- Conant, N.F. (1937) The occurrence of a human pathogenic fungus as a saprophyte in nature. Mycologia 29: 597–598. https://doi.org/10.1080/00275514.1937.12017229
- Crous, P.W., Wingfield, M.J., Burgess, T.I., Carnegie, A.J., Hardy, G.E.S.J., Smith, D., Summerell, B.A., Cano-Lira, J.F., Guarro, J., Houbraken, J., Lombard, L., Martín, M.P., Sandoval-Denis, M., Alexandrova, A.V., Barnes, C.W., Baseia, I.G., Bezerra, J.D.P., Guarnaccia, V., May, T.W., Hernández-Restrepo, M., Stchigel, A.M., Miller, A.N., Ordoñez, M.E., Abreu, V.P., Accioly, T., Agnello, C., Agustin Colmán, A., Albuquerque, C.C., Alfredo, D.S., Alvarado, P., Araújo-Magalhães, G.R., Arauzo, S., Atkinson, T., Barili, A., Barreto, R.W., Bezerra, J.L., Cabral, T.S., Camello Rodríguez, F., Cruz, R.H.S.F., Daniëls, P.P., da Silva, B.D.B., de Almeida, D.A.C., de Carvalho Júnior, A.A., Decock, C.A., Delgat, L., Denman, S., Dimitrov, R.A., Edwards, J., Fedosova, A.G., Ferreira, R.J., Firmino, A.L., Flores, J.A., García, D., Gené, J., Giraldo, A., Góis, J.S., Gomes, A.A.M., Gonçalves, C.M., Gouliamova, D.E., Groenewald, M., Guéorguiev, B.V., Guevara-Suarez, M., Gusmão, L.F.P., Hosaka, K., Hubka, V., Huhndorf, S.M., Jadan, M., Jurjević, Ž., Kraak, B., Kučera, V., Kumar, T.K.A., Kušan, I., Lacerda, S.R., Lamlertthon, S., Lisboa, W.S., Loizides, M., Luangsa-Ard, J.J., Lysková, P., Mac Cormack, W.P., Macedo, D.M., Machado, A.R., Malysheva, E.F., Marinho, P., Matočec, N., Meijer, M., Mešić, A., Mongkolsamrit, S., Moreira, K.A., Morozova, O.V., Nair, K.U., Nakamura, N., Noisripoom, W., Olariaga, I., Oliveira, R.J.V., Paiva, L.M., Pawar, P., Pereira, O.L., Peterson S,W., Prieto, M., Rodríguez-Andrade, E., Rojo De Blas, C., Roy, M., Santos, E.S., Sharma, R., Silva, G.A., Souza-Motta, C.M., Takeuchi-Kaneko, Y., Tanaka, C., Thakur, A., Smith, M.T., Tkalčec, Z., Valenzuela-Lopez, N., van der Kleij, P., Verbeken, A., Viana, M.G., Wang, X.W. & Groenewald, J.Z. (2017) Fungal Planet description sheets: 625-715. Persoonia 39: 270–467. https://doi.org/10.3767/persoonia.2017.39.11
- Di, M.S., Calzarano, F., Osti, F. & Mazzullo, A. (2004) Pathogenicity of fungi associated with a decay of kiwifruit. Australasian Plant Pathology 33: 337–342. https://doi.org/10.1071/AP04024
- Domsch, K.H., Gams, W. & Anderson, T.-H. (2007) Compendium of soil fungi. IHW-Verlag., Eching., 672 pp.
- Duran, P., Barra, P.J., Jorquera, M.A., Viscardi, S., Fernandez, C., Paz, C. & Bol, R. (2019) Occurrence of soil fungi in Antarctic pristine environments. Frontiers in Bioengineering and Biotechnology 7: 28. https://doi.org/10.3389/fbioe.2019.00028
- Ekanayaka, A.H., Hyde, K.D., Gentekaki, E., McKenzie, E.H.C., Zhao, Q., Bulgakov, T.S. & Camporesi, E. (2019) Preliminary classification of Leotiomycetes. Mycosphere 10: 310–489. https://doi.org/10.5943/mycosphere/10/1/7
- Gams, W. (2000) Phialophora and some similar morphologically little–differentiated anamorphs of divergent ascomycetes. Studies in Mycology 45: 187–199.
- Glass, N.L. & Donaldson, G.C. (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
- Halleen, F., Mostert, L. & Crous, P.W. (2007) Pathogenicity testing of lesser-known vascular fungi of grapevines. Australasian Plant Pathology 36: 277–285. https://doi.org/10.1071/AP07019
- Harrington, T.C. & McNew, D.L. (2003) Phylogenetic analysis places the Phialophora- like anamorph genus Cadophora in the Helotiales. Mycotaxon 87: 141–151.
- Hassan, N., Rafiq, M., Hayat, M., Shah, A.A. & Fariha, H. (2016) Psychrophilic and psychrotrophic fungi: a comprehensive review. Reviews in Environmental Science and Biotechnology 15: 147–172. https://doi.org/10.1007/s11157-016-9395-9
- Iliushin, V.A. (2020) First find of Cadophora antarctica Rodr.-Andrade, Stchigel, Mac Cormack & Cano in the Arctic. Czech Polar Reports 10: 147–152. https://doi.org/10.5817/CPR2020-2-11
- Iliushin, V.A., Kirtsideli, I.Y. & Vlasov, D.Y. (2022) Diversity of culturable microfungi of coal mine spoil tips in Svalbard. Polar Science 32: 100793. https://doi.org/10.1016/j.polar.2022.100793
- Johnston, P.R., Quijada, L., Smith, C.A., Baral, H.O., Hosoya, T., Baschien, C. & Townsend, J.P. (2019) A multigene phylogeny toward a new phylogenetic classification of Leotiomycetes. IMA Fungus 10: 1–22. https://doi.org/10.1186/s43008-019-0002-x
- Kelly, K.L. (1964) Inter-Society Color Council—National Bureau of Standards Color Name Charts Illustrated with Centroid Colors, Government Printing Office, Washington, DC, USA, 20 pp.
- Kirtsideli, I.Y., Vlasov, D.Y., Barantsevich, E.P., Krylenkov, V.A. & Sokolov, V.T. (2014) Microfungi from soil of polar island Izvestia Tsik (Kara sea). Mikologiya I Fitopatologiya 48: 365–371.
- Kirtsideli, I.Y., Vlasov, D.Y., Novozhilov, Y.K., Abakumov, E.V. & Barantsevich, E.P. (2018) Assessment of anthropogenic influence on Antarctic mycobiota in areas of Russian polar stations. Contemporary Problems of Ecology 11: 449–457. https://doi.org/10.1134/S1995425518050074
- Koukol, O. & Maciá-Vicente, J.G. (2022) Leptodophora gen. nov. (Helotiales, Leotiomycetes) proposed to accommodate selected root-associated members of the genus Cadophora. Czech Mycology 74: 57–66. https://doi.org/10.33585/cmy.74104
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. https://doi.org/10.1093/molbev/msy096
- Lagerberg, T., Lundberg, G. & Melin, E. (1927) Biological and practical researches into blueing in pine and spruce. Sven Skogsvardsforen Tidskr 25: 45–272.
- Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H., Valentin, F., Wallace, I.M., Wilm, A., Lopez, R., Thompson, J.D., Gibson, T.J. & Higgins, D.G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. https://doi.org/10.1093/bioinformatics/btm404
- Linnakoski, R., Kasanen, R., Lasarov, I., Marttinen, T., Oghenekaro, A.O., Sun, H., Asiegbu, F.O., Wingfield, M.J., Hantula, J. & Heliövaara, K. (2018) Cadophora margaritata sp. nov. and other fungi associated with the longhorn beetles Anoplophora glabripennis and Saperda carcharias in Finland. Antonie Van Leeuwenhoek 111: 2195–2211. https://doi.org/10.1007/s10482-018-1112-y
- Macia-Vicente, J.G., Piepenbring, M. & Koukol, O. (2020) Brassicaceous roots as an unexpected diversity hot-spot of helotialean endophytes. IMA Fungus 11: 16. https://doi.org/10.1186/s43008-020-00036-w
- Melin, E. & Nannfeldt, J.A. (1934) Svensk Skogsvårdsförening Tidskr 3–4: 407.
- Navarrete, F., Abreo, E., Martínez, S., Bettucci, L. & Lupo, S. (2011): Pathogenicity and molecular detection of Uruguayan isolates of Greeneria uvicola and Cadophora luteo-olivacea associated with grapevine trunk diseases. Phytopathologia Mediterranea 50: 166–175.
- Nováková, A., Hubka, V., Saiz-Jimenez, C. & Kolarik, M. (2012) Aspergillus baeticus sp. nov. and Aspergillus thesauricus sp. nov., two species in section Usti from Spanish caves. International Journal of Systematic and Evolutionary Microbiology 62: 2778–2785. https://doi.org/10.1099/ijs.0.041004-0
- O’Donnell, K. (1993) Fusarium and its near relatives. In: Reynolds, D.R. & Taylor, J.W. (Eds.) The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics. Wallingford, CABI Publishing, pp. 225–233.
- Pankova, I.G., Kirtsideli, I.Y., Iliushin, V.A., Zelenskaya, M.S., Vlasov, D.Y., Gavrilo, M.V. & Barantsevich, E.P. (2023) Diversity of Microfungi on Wood of the Coastal Zone of Heiss Island (Franz Joseph Land Archipelago). Mikologiya I Fitopatologiya 57: 184–197. https://doi.org/10.31857/S0026364823030091
- Pedersen, N.B., Matthiesen, H., Blanchette, R.A., Alfredsen, G., Held, B.W., Westergaard-Nielsen, A. & Hollesen, J. (2020) Fungal attack on archaeological wooden artefacts in the Arctic—Implications in a changing climate. Scientific Reports 5: 14577. https://doi.org/10.1038/s41598-020-71518-5
- Raper, K.B. & Fennell, D.I. (1965) The genus Aspergillus. Williams & Wilkins, Baltimore, 686 pp.
- Samson, R.A., Houbraken, J., Thrane, U., Frisvad, J.C. & Andersen, B. (2010) Food and indoor fungi, CBS Laboratory Manual Series 2. CBS-Fungal Biodiversity Centre, Utrecht, 390 pp.
- Samson, R.A., Visagie, C.M., Houbraken, J., Hong, S.B., Hubka, V., Klaassen, C.H.W., Perrone, G., Seifert, K.A., Susca, A., Tanney, J.B., Varga, J., Kocsubé, S., Szigeti, G., Yaguchi, T. & Frisvad, J.C. (2014) Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology 78: 141–173. https://doi.org/10.1016/j.simyco.2014.07.004
- Tamura, K. & Nei, M. (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
- Travadon, R., Lawrence, D.P., Rooney-Latham, S., Gubler, W.D., Wilcox, W.F., Rolshausen, P.E. & Baumgartner, K. (2015) Cadophora species associated with wood-decay of grapevine in North America. Fungal Biology 119: 53–66. https://doi.org/10.1016/j.funbio.2014.11.002
- Urbez-Torres, J.R., Haag, P., Bowen, P. & O’Gorman, D.T. (2014) Grapevine trunk diseases in British Columbia: incidence and characterization of the fungal pathogens associated with Esca and Petri Diseases of grapevine. Plant Disease 98: 469–482. https://doi.org/10.1094/PDIS-05-13-0523-RE
- Walsh, E., Duan, W., Mehdi, M., Naphri, K., Khiste, S., Scalera, A. & Zhang, N. (2018) Cadophora meredithiae and C. interclivum, new species from roots of sedge and spruce in a western Canada subalpine forest. Mycologia 110: 201–214. https://doi.org/10.1080/00275514.2017.1406748
- White, T.J., Bruns, T.D., Lee, S.B. & Taylor, J.W. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) PCR protocols: a guide to methods and applications. Academic Press, London, UK, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- Zhang, B., Li, X., Li, G., Wang Q.-M. & Wang, M. (2022) Cadophora species from marine glaciers in the Qinghai-Tibet Plateau: an example of unsuspected hidden biodiversity. IMA Fungus 13: 15. https://doi.org/10.1186/s43008-022-00102-5
- Zhang, T., Zhao, L., Yu, C., Wei, T. & Yu, L. (2017) Diversity and bioactivity of cultured aquatic fungi from the High Arctic region. Advances in Polar Science 28: 29–42.