Abstract
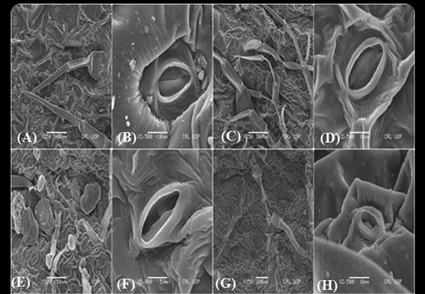
The foliar epidermal, stomatal and trichome properties are frequently used as plant microscopic attributes from a taxonomic or ecological perspective. Among the angiosperm families, the Lamiaceae is one of the most taxonomically difficult, economically significant, and medicinally important families. To better understand the foliar microanatomy of Lamiaceae taxa obtained from Northern Pakistan, this study attempts to determine the variational and diagnostic features of 27 species that were analyzed using light microscopy (LM) and scanning electron microscopy (SEM). UPGMA cluster analysis, principal component analysis (PCA) and semantic differential chart (SDC) were used to characterize, visualize, and compare the leaf micromorphology across all species. Three different types of epidermal cell types were examined on both foliar surfaces i.e., irregular, hexagonal, and polygonal. Anticlinal wall pattern also shows variation. Anomocytic stomata is dominant in 8 studied species followed by paractic in 5 species, anisocytic in 4 species and diacytic in 2 species. The trichomes could be broadly classified into two groups, glandular trichomes (GTs) and non-glandular trichomes (NGTs). Lamiaceae has two types of glandular trichomes, small capitate and large peltate, which differ in size, structure, and distribution. In present research five distinct forms of non-glandular trichomes are unicellular, multicellular, stellate, branched, and unbranched trichomes. The results that characterized, illustrated, and contrasted the leaf anatomical characteristics of the Lamiaceae taxa can be better understood with the aid of statistical analysis. Future research will depend on these findings to improve the systematics of Lamiaceae taxa. These characteristics have a large taxonomic potential when considered as a whole.
References
- Abdullah, M., Ullah, Z., Shah, S.M.I., Ahmad, M., Khan, A., Shah, H., Ullah, A., Ahmad, S., Albeshr, M.F., Emmanuel, O. & Ullah, H. (2024) Systematic implication of trichome morphology in alpine flora from Hindukush Mountain range. Genetic Resources and Crop Evolution 2024. https://doi.org/10.1007/s10722-024-02079-z
- Abu-Asab, M.S. & Cantino, P.D. (1987) Phylogenetic implications of leaf anatomy in subtribe Melittidinae (Labiatae) and related taxa. Journal of the Arnold Arboretum 68: 1–34. https://doi.org/10.5962/p.185940
- Akinsulire, O.P., Oladipo, O.T., Akinkunmi, O.C., Adeleye, O.E. & Adelalu, K.F. (2020) Leaf and Petiole Micro-Anatomical Diversities in Some Selected Nigerian Species of Loefl.: the Significance in Species Identification at Vegetative State. Acta Biologica Marisiensis 3 (1): 15–29. https://doi.org/10.2478/abmj-2020-0002
- Al-Shehbaz, I., Beilstein, M. & Kellogg, E. (2006) Systematics and phylogeny of the Brassicaceae (Cruciferae): an overview. Plant systematics and evolution 259: 89–120. https://doi.org/10.1007/s00606-006-0415-z
- Amelunxen, F. (1964) Elektronenmikroskopische Untersuchungen an den Drüsenhaaren von Mentha piperita L. Planta medica 12 (02): 121–139. https://doi.org/10.1055/s-0028-1100158
- Amelunxen, F., Wahlig, T. & Arbeiter, H. (1969) Über den Nachweis des ätherischen Öls in isolierten Drüsenhaaren und Drüsenschuppen von Mentha piperita L. Zeitschrift für Pflanzenphysiologie 61 (1): 68–72.
- Arceo, A., Tham, M., Guven, G., MacLean, H.L. & Saxe, S. (2021) Capturing variability in material intensity of single-family dwellings: A case study of Toronto, Canada. Resources, Conservation and Recycling 175: 105885. https://doi.org/10.1016/j.resconrec.2021.105885
- Ascensao, L., Marques, N. & Pais, M.S. (1997) Peltate glandular trichomes of Leonotis leonurus leaves: ultrastructure and histochemical characterization of secretions. International Journal of Plant Sciences 158 (3): 249–258. https://doi.org/10.1086/297436
- Ascensão, L., Mota, L., DE, M. & Castro, M. (1999) Glandular Trichomes on the Leaves and Flowers of Plectranthus ornatus: Morphology, Distribution and Histochemistry. Annals of Botany 84 (4): 437–447. https://doi.org/10.1006/anbo.1999.0937
- Ascensão, L. & Pais, M. (1998) The leaf capitate trichomes of Leonotis leonurus: Histochemistry, Ultrastructure and Secretion. Annals of Botany 81 (2): 263–271. https://doi.org/10.1006/anbo.1997.0550
- Ashfaq, S., Ahmad, M., Zafar, M., Sultana, S., Bahadur, S., Ullah, F., Zaman, W., Ahmed, S.N. & Nazish, M. (2019) Foliar micromorphology of Convolvulaceous species with special emphasis on trichome diversity from the arid zone of Pakistan. Flora 255: 110–124. https://doi.org/10.1016/j.flora.2019.04.007
- Atalay, Z., Celep, F., Bara, F. & Doğan, M. (2016) Systematic significance of anatomy and trichome morphology in Lamium (Lamioideae; Lamiaceae). Flora - Morphology, Distribution, Functional Ecology of Plants 225: 60–75. https://doi.org/10.1016/j.flora.2016.10.006
- Azizian, D. & Cutler, D. (1982) Anatomical, cytological and phytochemical studies on Phlomis L. and Eremostachys Bunge (Labiatae). Botanical journal of Linnaean Society 85 (4): 249–281. https://doi.org/10.1111/j.1095-8339.1982.tb00373.x
- Badry, M.O., Osman, A.K., Aboulela, M., Gafar, S. & Nour, I.H. (2024) Taxonomic implications of normal and abnormal stomatal complexes in Indigofera L. (Indigofereae, Faboideae, Fabaceae). Protoplasma 261: 991–1021. https://doi.org/10.1007/s00709-024-01951-0
- Beilstein, M.A., Al‐Shehbaz, I.A. & Kellogg, E.A. (2006) Brassicaceae phylogeny and trichome evolution. American Journal of Botany 93 (4): 607–619. https://doi.org/10.3732/ajb.93.4.607
- Belhadj, S., Derridj, A., Aigouy, T., Gers, C., Gauquelin, T. & Mevy, J.P. (2007) Comparative morphology of leaf epidermis in eight populations of Atlas pistachio (Pistacia atlantica Desf., Anacardiaceae). Microscopy Research and Technique 70 (10): 837–846. https://doi.org/10.1002/jemt.20483
- Benjamin, E.J., Virani, S.S., Callaway, C.W., Chamberlain, A.M., Chang, A.R., Cheng, S., Chiuve, S.E., Cushman, M., Delling, F.N., Deo, R., de Ferranti, S.D., Ferguson, J.F., Fornage, M., Gillespie, C., Isasi, C.R., Jiménez, M.C., Jordan, L.C., Judd, S.E., Lackland, D., Lichtman, J.H., Lisabeth, L., Liu, S., Longenecker, C.T., Lutsey, P.L., Mackey, J.S., Matchar, D.B., Matsushita, K., Mussolino, M.E., Nasir, K., O’Flaherty, M., Palaniappan, L.P., Pandey, A., Pandey, D.K., Reeves, M.J., Ritchey, M.D., Rodriguez, C.J., Roth, G.A., Rosamond, W.D., Sampson, U.K.A., Satou, G.M., Shah, S.H., Spartano, N.L., Tirschwell, D.L., Tsao, C.W., Voeks, J.H., Willey, J.Z., Wilkins, J.T., Wu, J.H.Y., Alger, H.M., Wong, S.S. & Muntner, P. (2018) Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 137 (12): e67–e492. https://doi.org/10.1161/CIR.0000000000000558
- Bhatt, A., Naidoo, Y. & Nicholas, A. (2010) The foliar trichomes of Plectranthus laxiflorus Benth [Lamiaceae]: an important medicinal plant. New Zealand Journal of Botany 48 (2): 55–61. https://doi.org/10.1080/0028825X.2010.482958
- Bini Maleci, L. & Servettaz, O. (1991) Morphology and distribution of trichomes in Italian species of Teucrium sect. Chamaedrys (Labiatae)—a taxonomical evaluation. Plant Systematics & Evolution 174: 83–91. https://doi.org/10.1007/BF00937696
- Abu-Asab, M.S. & Cantino, P.D. (1987) Phylogenetic implications of leaf anatomy in subtribe Melittidinae (Labiatae) and related taxa. Journal of the Arnold Arboretum 68: 1–34.
- https://doi.org/10.5962/p.185940
- Bosabalidis, A. (1990) Glandular trichomes in Satureja thymbra leaves. Annales Botany 65 (1): 71–78. https://doi.org/10.1093/oxfordjournals.aob.a087910
- Bosabalidis, A. & Tsekos, I. (1982) Ultrastructural studies on the secretory cavities of Citrus deliciosa Ten. II. Development of the essential oil-accumulating central space of the gland and process of active secretion. Protoplasma 112: 63–70. https://doi.org/10.1007/BF01280216
- Bosabalidis, A. & Tsekos, I. (1984) Glandular hair formation in Origanum species. Annalas of Botany 53 (4): 559–563. https://doi.org/10.1093/oxfordjournals.aob.a086719
- Botanica, A. (2009) Micromorphological studies of Lallemantia L. (Lamiaceae) species growing in Turkey. Acta Biologica Cracoviensia Series Botanica 51 (1): 45–54.
- Briquet, J. (1895) Notes sur la flore du massif de Platé. R. Burkhardt. https://doi.org/10.3406/globe.1895.1984
- Candido, D.S., Claro, I.M., De Jesus, J.G., Souza, W.M., Moreira, F.R., Dellicour, S., Mellan, T.A., Du Plessis, L., Pereira, R.H. & Sales, F.C. (2020) Evolution and epidemic spread of SARS-CoV-2 in Brazil. Science 369 (6508): 1255–1260. https://doi.org/10.1126/science.abd2161
- Cantino, P.D. (1990) The phylogenetic significance of stomata and trichomes in the Labiatae and Verbenaceae. Journal of Arnold Arboretum 71: 323–370. https://doi.org/10.5962/p.184532
- Casella, F., Vurro, M., Valerio, F., Perrino, E.V., Mezzapesa, G.N. & Boari, A. (2023) Phytotoxic Effects of Essential Oils from Six Lamiaceae Species. Agronomy 13: 257. https://doi.org/10.3390/agronomy13010257
- Celep, F., Kahraman, A., Atalay, Z., Dogan, M. (2011) Morphology, anatomy and trichome properties of’Lamium truncatum’Boiss.(Lamiaceae) and their systematic implications. Australian Journal of Crop Sciences. 5 (2): 147–153.
- Clarke, J. (1960) Preparation of leaf epidermis for topographic study. Stain Technology 35 (1): 35–39. https://doi.org/10.3109/10520296009114713
- Cousins, D.J. (Ed.) (1994) Medicinal, essential oil, culinary herb and pesticidal plants of the Labiatae 1973-1993. CAB International, London, 354 pp.
- de Oliveira, A.B., de Mendonça, M.S. & Meira, R.M. (2013) Anatomy of vegetative organs of Scutellaria agrestis, a medicinal plant cultivated by riverine populations of the Brazilian Amazon. Revista Brasileira de Farmacognosi 23 (3): 386–397. https://doi.org/10.1590/S0102-695X2013005000034
- Dehshiri, M., Azadbakht, M. (2012) Anatomy of Iranian species Teucrium polium (Lamiaceae). Journal of Biology and Today’s World 1 (2): 93–98.
- Dudai, N., Werker, E., Putievsky, E., Ravid, U., Palevitch, D. & Halevy, A. (1988) Glandular hairs and essential oils in the leaves and flowers of Majorana syriaca. Israel Journal of Botany 37 (1): 11–18.
- Erdtman, G. (1945) Pollen morphology and plant taxonomy. Svensk botanisk tidskrift 38: 163–168.
- Fajuke, A.A., Makinde, A.M., Oloyede, F.A. & Akinloye, J.A. (2018) Comparative epidermal anatomical studies in six taxa of genus Nephrolepis Swart in Nigeria. Tropical Plant Reseearh 5 (1): 19. https://doi.org/10.22271/tpr.2018.v5.i1.004
- Gairola, S., Naidoo, Y., Bhatt, A. & Nicholas, A. (2009) An investigation of the foliar trichomes of Tetradenia riparia (Hochst.) Codd [Lamiaceae]: an important medicinal plant of Southern Africa. Flora-Morphology, Distribution, Functional Ecology of Plants 204 (4): 325–330. https://doi.org/10.1016/j.flora.2008.04.002
- Gerçek, Y.C., Şahin, A.A., Bayram, N.E., Çelik, S., Sefalı, A., Gıdık, B., Öz, G.C. & Pınar, N.M. (2022) Anatomy, trichome micromorphology and phytochemical profile of Stachys rizeensis R.Bhattacharjee from Turkey. South africa journal of botany 149: 19–28. https://doi.org/10.1016/j.sajb.2022.05.046
- Gul, S., Ahmad, M., Zafar, M., Bahadur, S., Sultana, S., Ashfaq, S., Ullah, F., Kilic, O., Hassan, Fu. & Siddiq, Z. (2019) Foliar epidermal anatomy of Lamiaceae with special emphasis on their trichomes diversity using scanning electron microscopy. Microscopy reearch & technique 82 (3): 206–223. https://doi.org/10.1002/jemt.23157
- Hanlidou, E., Kokkini, S., Bosabalidis, A. & Bessière, J-M. (1991) Glandular trichomes and essential oil constituents of Calamintha menthifolia (Lamiaceae). Plant Systematics and Evolution 177: 17–26. https://doi.org/10.1007/BF00937823
- Haruna, H. & Ashir, H. (2017) leaf epidermal members of the. Journal of Pure & Applied Sciences 10 (1): 670–675.
- Heinrich, G., Schultze, W., Pfab, I. & Boettger, M. (1983) The site of essential oil biosynthesis in Poncirus trifoliata and Monarda fistulosa. Physiologie Vegetale 21: 257–268.
- Herman, P. (1998) The leaf anatomy of two Clerodendrum species (Verbenaceae). South African Journal of Botany 64 (4): 246–249. https://doi.org/10.1016/S0254-6299(15)30888-7
- Husain, S., Marin, P., Diklic, N. & Patcovic, B. (1989) micromorphological and phytochemical studies in. Pakistan Journal of Botany 21 (2): 210–217.
- Inamdar, J. & Bhatt, D. (1972a) Epidermal structure and ontogeny of stomata in vegetative and reproductive organs of Ephedra and Gnetum. Annals of Botany 36 (5): 1041–1046. https://doi.org/10.1093/oxfordjournals.aob.a084647
- Inamdar, J. & Bhatt, D. (1972b) Structure and development of stomata in some Labiatae. Annal Botany 36 (2): 335–344. https://doi.org/10.1093/oxfordjournals.aob.a084593
- James, O.E., Green, B.O., Ajuru, M.G. & Wilson, V. (2021) Foliar epidermal anatomy and its taxonomic implications within the family Euphorbiaceae in The Niger Delta region of Nigeria. Journal of Frontiers in Life Science Research 1 (1): 48–55. https://doi.org/10.53294/ijflsr.2021.1.1.0036
- Jurišić, G.R., Vladimir-Knežević, S., Kremer, D., Kalodera, Z. & Vuković, J. (2007) Trichome micromorphology in Teucrium (Lamiaceae) species growing in Croatia. Biologia 62: 148–156. https://doi.org/10.2478/s11756-007-0023-6
- Kahraman, A., Celep, F. & Dogan, M. (2010a) Anatomy, trichome morphology and palynology of Salvia chrysophylla Stapf (Lamiaceae). South African Journal of Botany 76 (2): 187–195. https://doi.org/10.1016/j.sajb.2009.10.003
- Kahraman, A., Dogan, M., Celep, F., Akaydin, G. & Koyuncu, M.J.NJ.O.B. (2010b) Morphology, anatomy, palynology and nutlet micromorphology of the rediscovered Turkish endemic Salvia ballsiana (Lamiaceae) and their taxonomic implications. Nordic Journal of Botany 28 (1): 91–99. https://doi.org/10.1111/j.1756-1051.2009.00384.x
- Karabourniotis, G., Liakopoulos, G., Nikolopoulos, D. & Bresta, P. (2020) Protective and defensive roles of non-glandular trichomes against multiple stresses: structure-function coordination. Journal of Forestry Research 31 (1): 1–12. https://doi.org/10.1007/s11676-019-01034-4
- Kaya, A., Demirci, B. & Baser, K. (2007) Micromorphology of glandular trichomes of Nepeta congesta Fisch. & Mey. var. congesta (Lamiaceae) and chemical analysis of the essential oils. South Africa Journal of Botany 73 (1): 29–34. https://doi.org/10.1016/j.sajb.2006.05.004
- Khan, R., Abidin, S.Z.U., Ahmad, M., Zafar, M., Liu, J., Jamshed, S. & Kiliç, Ö. (2019) Taxonomic importance of SEM and LM foliar epidermal micro-morphology: A tool for robust identification of gymnosperms. Flora 255: 42–68. https://doi.org/10.1016/j.flora.2019.03.016
- Klich, M.G. (2000) Leaf variations in Elaeagnus angustifolia related to environmental heterogeneity. Environmental and Experimental Botany 44 (3): 171–183. https://doi.org/10.1016/S0098-8472(00)00056-3
- Liakoura, V., Stavrianakou, S., Liakopoulos, G., Karabourniotis, G. & Manetas, Y. (1999) Effects of UV-B radiation on Olea europaea: comparisons between a greenhouse and a field experiment. Tree physiology 19 (13): 905–908. https://doi.org/10.1093/treephys/19.13.905
- Maffei, M. & Codignola, A. (1990) Photosynthesis, photorespiration and herbicide effect on terpene production in peppermint (Mentha piperita L.). Journal of Essential Oil Research 2 (6): 275–286. https://doi.org/10.1080/10412905.1990.9697886
- Mannethody, S. & Purayidathkandy, S. (2018) Trichome micromorphology and its systematic significance in Asian Leucas (Lamiaceae). Flora 242: 70–78. https://doi.org/10.1016/j.flora.2018.03.007
- Marin, P.D., Petković, B. & Duletić, S. (1994) Nutlet sculpturing of selected Teucrium species (Lamiaceae): a character of taxonomic significance. Plant Systematics Evolution 192: 199–214. https://doi.org/10.1007/BF00986252
- Metcalfe, C.R., (1968) Current developments in systematic plant anatomy. In: Heywood, V.H. (Ed.) Modern methods in plant taxonomy. Academic Press, London & New York, pp. 45–57.
- Stefanaki, A. & van Andel, T. (2021) Mediterranean aromatic herbs and their culinary use. In: Aromatic Herbs in Food. Academic Press, pp. 93–121. https://doi.org/10.1016/B978-0-12-822716-9.00003-2
- Mohd Rozar, N., Sidik, M.H., Razik, M.A., Ahmad, Kamaruddin, S., Rozar, M.K.A.M., Usman, I. & Alown, B.E. (2023) A hierarchical cluster analysis of port performance in Malaysia. Maritime Business Review 8 (3): 194–208. https://doi.org/10.1108/MABR-07-2020-0040
- Moon, H-K., Hong, S-P., Smets, E. & Huysmans, S. (2009) Phylogenetic significance of leaf micromorphology and anatomy in the tribe Mentheae (Nepetoideae: Lamiaceae). Botanical Journal of Linnaean Society 160 (2): 211–231. https://doi.org/10.1111/j.1095-8339.2009.00979.x
- Mun, H.G. & Hong, SP (2003) The taxonomic consideration of leaf epidermal microstructure in Lycopus L. (Mentheae, Lamiaceae). Korean Journal of Plant Taxonomy 33 (2): 151–164. https://doi.org/10.11110/kjpt.2003.33.2.151
- Navarro, T. & El Oualidi, J. (1999) Trichome morphology in Teucrium L.(Labiatae). A taxonomic review. Anales del Jardín Botánico de Madrid 2: 277–297. https://doi.org/10.3989/ajbm.1999.v57.i2.203
- Navarro, T. & El Oualidi, J. (2000) Synopsis of Teucrium L.(Labiatae) in the Mediterranean region and surrounding areas. Flora Mediterranea 10: 349–363.
- Nazir, M.S., Wahjoedi, B.A., Yussof, A.W. & Abdullah, M.A.J.B. (2013) Eco-friendly extraction and characterization of cellulose from oil palm empty fruit bunches. Bioresource 8 (2): 2161–2172. http://dx.doi.org/10.15376/biores.8.2.2161-2172
- Nazish, M., Zafar, M., Ahmad, M., Sultana, S., Ullah, R., Alqahtani, A.S., Ullah, F., Ahmad, S., Ashfaq, S. & Ullah, F. (2019) Palyno‐morphological investigations of halophytic taxa of Amaranthaceae through SEM from Salt range of Northern Punjab, Pakistan. Microscopy Research & Technique 82 (3): 304–316. https://doi.org/10.1002/jemt.23173
- Osman, A. (2012) Trichome micromorphology of Egyptian Ballota (Lamiaceae) with emphasis on its systematic implication. Pakistan Journal of Botany 44 (1): 33–46
- Perrino, E.V., Mahmoud, Z.N.A., Valerio, F., Tomaselli, V., Wagensommer, R.P. & Trani, A. (2023) Synecology of Lagoecia cuminoides L. in Italy and evaluation of functional compounds presence in its water or hydroalcoholic extracts. Scientific Reports 13: 20906. https://doi.org/10.1038/s41598-023–48065-w
- Perveen, A. & Qaiser, M.J.P.J.B. (2004) Pollen morphology of the family Labiatae from Pakistan. Pakistan Journal of Botany 35 (5): 671–694.
- Putievsky, E., Ravid, U. & Dudai, N. (1988) Phenological and seasonal influences on essential oil of a cultivated clone of Origanum vulgare L. Journal of the Science of Food and Agriculture 43 (3): 225–228. https://doi.org/10.1002/jsfa.2740430304
- Rashid, N., Zafar, M., Ahmad, M., Khan, M.A., Malik, K., Sultana, S. & Shah, S.N. (2019) Taxonomic significance of leaf epidermis in tribe Trifolieae L. (Leguminosae; Papilionoideae) in Pakistan. Plant Biosystem 153 (3): 406–416. https://doi.org/10.1080/11263504.2018.1492995
- Raza, J., Ahmad, M., Zafar, M., Yaseen, G., Sultana, S. & Majeed, S. (2022) Systematic significance of seed morphology and foliar anatomy among Acanthaceous taxa. Biologia 77 (11): 3125–3142. https://doi.org/10.1007/s11756-022-01137-0
- Rozar, N.M., Sidik, M.H., Razik, M.A., Kamaruddin, S.A., Rozar, M.K.A.M., Usman, I. & Alown, B.E. (2022) A hierarchical cluster analysis of port performance in Malaysia. Maritime Business Review 8 (3): 194–208. https://doi.org/10.1108/MABR-07-2020-0040
- Rudall, P. (1980) Leaf anatomy of the subtribe Hyptidinae (Labiatae). Botanical journal of Linnaean Society 80 (4): 319–340. https://doi.org/10.1111/j.1095-8339.1980.tb01667.x
- Salmaki, Y., Zarre, S., Jamzad, Z. & Bräuchler, C. (2009) Trichome micromorphology of Iranian Stachys (Lamiaceae) with emphasis on its systematic implication. Flora-Morphology, Distribution, Functional Ecology of Plants 204 (5): 371–381. https://doi.org/10.1016/j.flora.2008.11.001
- Sanoj, E. & Deepa, P. (2021) Micromorphological variations of trichomes in the genus Ocimum L. Plant science today 8 (3): 429–436. https://doi.org/10.14719/pst.2021.8.3.1006
- Satil, F., Ünal, M. & Hopa, E. (2007) Comparative morphological and anatomical studies of Hymenocrater bituminosus Fisch. & CA Mey.(Lamiaceae) in Turkey. Turkish Journal of Botany 31 (3): 269–275.
- Schilmiller, A.L., Last, R.L. & Pichersky, E. (2008) Harnessing plant trichome biochemistry for the production of useful compounds. The Plant Journal 54 (4): 702–711. https://doi.org/10.1111/j.1365-313X.2008.03432.x
- Schreuder, M.D., Brewer, C.A. & Heine, C. (2001) Modelled influences of non-exchanging trichomes on leaf boundary layers and gas exchange. Journal of Theoretical Biology 210 (1): 23–32. https://doi.org/10.1006/jtbi.2001.2285
- Serrato-Valenti, G., Bisio, A., Cornara, L. & Ciarallo, G. (1997) Structural and histochemical investigation of the glandular trichomes of Salvia aurea L. leaves, and chemical analysis of the essential oil. Annales Botany 79 (3): 329–336. https://doi.org/10.1006/anbo.1996.0348
- Shah, S.N., Ahmad, M., Zafar, M., Malik, K., Rashid, N., Ullah, F., Zaman, W. & Ali, M. (2018) A light and scanning electron microscopic diagnosis of leaf epidermal morphology and its systematic implications in Dryopteridaceae: Investigating 12 Pakistani taxa. Micron 111: 36–49. https://doi.org/10.1016/j.micron.2018.05.008
- Shah G, Naidu, A. (1983) Trichomes on leaves of some Lamiaceae. Geophytologist 13 (2): 165–176.
- Sharma, K.K. (2006) Optics: principles and applications. Elsevier. Academic Press, 656 pp.
- El‐Gazzar, A. & Watson, L. (1970) A taxonomic study of Labiatae and related genera. New Phytologist 69 (2): 451–486. https://doi.org/10.1111/j.1469-8137.1970.tb02443.x
- Stant, M.Y. (1973) The role of the scanning electron microscope in plant anatomy. Kew Bulletin 28: 105–115. https://doi.org/10.2307/4117068
- Frezza, C., Venditti, A., Serafini, M. & Bianco, A. (2019) Phytochemistry, chemotaxonomy, ethnopharmacology, and nutraceutics of Lamiaceae. Studies in natural products chemistry 62: 125–178. https://doi.org/10.1016/B978-0-444-64185-4.00004-6
- Sufyan, M., Badshah, I., Ahmad, M., Zafar, M., Bahadur, S. & Rashid, N. (2018) Identification of medicinally used Flora using pollen features imaged in the scanning electron microscopy in the lower Margalla Hills Islamabad Pakistan. Microscopy and Microanalysis 24 (3): 292–299. https://doi.org/10.1017/S1431927618000326
- Tissier, A. (2012) Glandular trichomes: what comes after expressed sequence tags? The Plant Journal 70 (1): 51–68. https://doi.org/10.1111/j.1365-313X.2012.04913.x
- Ullah, F., Zafar, M., Ahmad, M., Dilbar, S., Shah, S.N., Sohail, A., Zaman, W., Iqbal, M., Bahadur, S. & Tariq, A. (2018) Pollen morphology of subfamily Caryophylloideae (Caryophyllaceae) and its taxonomic significance. Microscopy Research and Technique 81 (7): 704–715. https://doi.org/10.1002/jemt.23026
- Vrachnakis, T. (2003) Trichomes of Origanum dictamnus L. (Labiatae). Phyton; Annales Rei Botanicae 43 (1): 109–133.
- Wagner, G., Wang, E. & Shepherd, R. (2004) New Approaches for Studying and Exploiting an Old Protuberance, the Plant Trichome. Annals of Botany 93 (1): 3–11. https://doi.org/10.1093/aob/mch011
- Webster, G., Del-Arco-Aguilar, M. & Smith, B. (1996) Systematic distribution of foliar trichome types in Croton (Euphorbiaceae). Botanical journal of linnaean society 121 (1): 41–57. https://doi.org/10.1006/bojl.1996.0023
- Werker, E. (1993) Function of essential oil‐secreting glandular hairs in aromatic plans of Lamiacea—a review. Flavour and Fragrance Journal 8 (5): 249–255. https://doi.org/10.1002/ffj.2730080503
- Werker, E., Ravid, U. & Putievsky, E. (1985) Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae. Isrrael Journal of Plant Sciences 34 (1): 31–45.
- Zaman, W., Ullah, F., Parmar, G., Saqib, S., Ayaz, A. & Park, S. (2022a) Foliar micromorphology of selected medicinal Lamiaceae taxa and their taxonomic implication using scanning electron microscopy. Microscopy Research and Technique 85 (9): 3217–3236. https://doi.org/10.1002/jemt.24179