Abstract
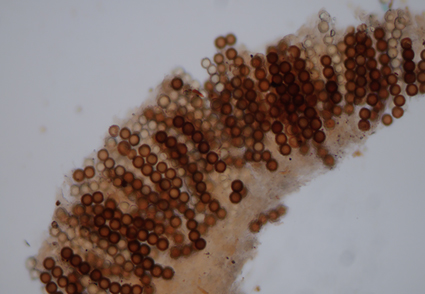
Hydnotrya qinghaiensis, a new species, is described from Tibetan Plateau, China. It is characterized by the irregular globose, brownish and large ascomata. Morphologically, the new species differs from the closely related H. cerebriformis and H. variiformis by the color, size, and shape of the ascocarp and the shape of ascospores. A detailed description and comparison with closely related species are provided, and the phylogenetic placement of the new species is also discussed based on the nuclear internal transcribed spacer region (ITS).
References
- Akaike, H. (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723. https://doi.org/10.1109/TAC.1974.1100705
- Berkeley, M.J. (1844) XLII. —Notices of British fungi. Journal of Natural History 13: 340–360. https://doi.org/10.1080/03745484409442617
- Berkeley, M.J. & Broome, C.E. (1846) IX.—Notices of British hypogœous fungi. The Annals and Magazine of Natural History 18: 73–82. https://doi.org/10.1080/037454809496568
- Bessey, E. & Thompson, B.E. (1920) An undescribed Genea from Michigan. Mycologia 12: 282–285. https://doi.org/10.2307/3753194
- Brock, P.M., Döring, H. & Bidartondo, M.I. (2009) How to know unknown fungi: the role of a herbarium. New Phytologist 181: 719–724. https://doi.org/10.1111/j.1469-8137.2008.02703.x
- Cao, J.Z., Fan, L. & Liu, B. (1990) Notes on the genus Gyromitra from China. Acta Mycologica Sinica 9: 100–108.
- Cox, F., Barsoum, N., Lilleskov, E.A. & Bidartondo, M.I. (2010) Nitrogen availability is a primary determinant of conifer mycorrhizas across complex environmental gradients. Ecology Letters 13: 1103–1113. https://doi.org/10.1111/j.1461-0248.2010.01494.x
- Dzhagan, V., Alvarado, P. & Shcherbakova, Y. (2015) Hydnotrya bailii Soehner (Ascomycota, Pezizales), a new hypogeous fungus for the Ukraine. Nova Hedwigia 100: 259–263. https://doi.org/10.1127/0029-5035/2014/0182
- Fischer, E. (1898) Bemerkungen über Geopora und verwandte Hypogaeen. Hedwigia 37: 56–60.
- Fries, E.M. (1849) Summa Vegetabilium Scandinaviae. Holmiae et Lipsiae, pp. 259–572.
- Fu, C.H., Tsai, M.J., Chang, T.T., Lin, C.L., Wong, K.F. & Li, H.T. (2018) First report of Hydnotrya tulasnei in central Taiwan. Taiwan Journal of Forest Science 33: 245–250.
- Gilkey, H.M. (1939) Tuberales of North America. Oregon State College, Oregon, 64 pp.
- Gilkey, H.M. (1947) New or otherwise noteworthy species of Tuberales. Mycologia 39: 441–452. https://doi.org/10.1080/00275514.1947.12017626
- Hall, F.R. (1999) Precision farming: Technologies and information as risk-reduction tools. ACS Symposium Series 734: 96–116. https://doi.org/10.1021/bk-1999-0734.ch008
- Harkness, H.W. (1899) Californian hypogeous fungi. Proceedings of the California Academy of Sciences Third Series 1: 241–292.
- Hart, B.T.N., Smith, J.E., Luoma, D.L. & Hatten, J.A. (2018) Recovery of ectomycorrhizal fungus communities fifteen years after fuels reduction treatments in ponderosa pine forests of the Blue Mountains, Oregon. Forest Ecology and Management 422: 11–22. https://doi.org/10.1016/j.foreco.2018.03.050
- Izzo, A.D., Meyer, M., Trappe, J.M., North, M. & Bruns, T.D. (2005) Hypogeous ectomycorrhizal fungal species on roots and in small mammal diet in a mixed-conifer forest. Forest Science 51: 243–254. https://doi.org/10.1093/forestscience/51.3.243
- Karsten, P.A. (1879) Rysslands, Finlands och den Skandinaviska halföns Hattsvampar. Förra Delen: Skifsvampar. Bidrag till Kännedom av Finlands Natur och Folk 32: 1–571.
- Kranabetter, J.M., Stoehr, M. & O’Neill, G.A. (2015) Ectomycorrhizal fungal maladaptation and growth reductions associated with assisted migration of Douglas-fir. New Phytologist 206: 1135–1144. https://doi.org/10.1111/nph.13287
- Læssøe, T. & Hansen, K. (2007) Truffle trouble: what happened to the Tuberales? Mycological Research 111: 1075–1099. https://doi.org/10.1016/j.mycres.2007.08.004
- Li, L., Zhao, Y.C., Zhang, X.L., Su, H.Y., Li, S.H. & Zhou, D.Q. (2013) Hydnotrya laojunshanensis sp. nov. from China. Mycotaxon 125: 277–282. https://doi.org/10.5248/125.277
- Nylander, J.A., Ronquist, F., Huelsenbeck, J.P. & Nieves-Aldrey, J.L. (2004) Bayesian phylogenetic analysis of combined data. Systematic Biology 53: 47–67. https://doi.org/10.1080/10635150490264699
- O’Donnell, K., Cigelnik, E., Weber, N.S. & Trappe, J.M. (1997) Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. Mycologia 89: 48–65. https://doi.org/10.1080/00275514.1997.12026754
- Osmundson, T.W., Robert, V.A., Schoch, C.L., Baker, L.J., Smith, A., Robich, G., Mizzan, L. & Garbelotto, M.M. (2013) Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. PloS One 8: e62419. https://doi.org/10.1371/journal.pone.0062419
- Piña-Páez, C., Garibay-Orijel, R., Guevara-Guerrero, G. & Castellano, M.A. (2017) Descripción y distribución de Hydnotrya cerebriformis (Discinaceae: Pezizales) en México. Revista Mexicana de Biodiversidad 88: 269–274. https://doi.org/10.1016/j.rmb.2017.03.017
- Reithmeier, L. & Kernaghan, G. (2013) Availability of ectomycorrhizal fungi to black spruce above the present treeline in Eastern Labrador. PLoS ONE 8: e77527. https://doi.org/10.1371/journal.pone.0077527
- Ronquist, F., Teslenko, M., Van Der Mark. P., Ayres, D.L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Schaeffer, J.C. (1774) Fungorum qui in Bavaria et Palatinatu circa Ratisbonam nascuntur icones, nativis coloribus expressae III et IV. Erlangen: J.J. Palm.
- Slavova, M., Assyov, B., Denchev, T.T. & Denchev, C.M. (2021) Hydnotrya michaelis–an uncommon fungus from unexpected habitat. Ecologia Balkanica 13: 167–171.
- Soehner E (1959) Tuberaceen Studien V. Mitteilungen der Botanischen Staatssammlung München.
- Spooner, B. (1992) A new species of Hydnotrya (Helvellaceae) from the British Isles. Kew Bulletin 47: 502. https://doi.org/10.2307/4110576
- Stamatakis, A. (2006) Phylogenetic models of rate heterogeneity: a high performance computing perspective. Proceedings 20th IEEE International Parallel & Distributed Processing Symposium: 8. https://doi.org/10.1109/IPDPS.2006.1639535
- Stielow, B., Bubner, B., Hensel, G., Münzenberger, B., Hoffmann, P., Klenk, H.-P. & Göker, M. (2010) The neglected hypogeous fungus Hydnotrya bailii Soehner (1959) is a widespread sister taxon of Hydnotrya tulasnei (Berk.) Berk. & Broome (1846). Mycological Progress 9: 195–203. https://doi.org/10.1007/s11557-009-0625-1
- Svrček, M. (1955) Co jest Hydnotrya carnea (Corda) Zobel? Několik zajimavých druhů našich větších vřeckatých hub, pp. 185–189.
- Tao, K. & Liu, B. (1989). Preliminary study on Hydnotrya from China. Journal of Shanxi University 12: 81–85.
- Tedersoo, L., Hansen, K., Perry, B.A. & Kjøller, R. (2006) Molecular and morphological diversity of pezizalean ectomycorrhiza. New Phytologist 170: 581–596. https://doi.org/10.1111/j.1469-8137.2006.01678.x
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. & Higgins, D.G. (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 24: 4876–4882. https://doi.org/10.1093/nar/25.24.4876
- Trappe, J.M. (1975) Generic synonyms in the Tuberales. Mycotaxon 2: 109–122.
- Trappe, J.M. & Castellano, M.A. (2000) New sequestrate Ascomycota and Basidiomycota covered by the Northwest Forest Plan. Mycotaxon 75: 153–179.
- Tulasne, L.R. & Tulasne, C. (1843) Champignons hypogés de la famille des Lycoperdacées, observés dans les environs de Paris et les départements de la Vienne et d’lndre-et-Loire. Annales Des Sciences Naturelles Série 2: 373–381.
- Vohnik, M., Fendrych, M., Kolarik, M., Gryndler, M., Hrrselova, H., Albrechtova, J. & Vosatka, M. (2007) The ascomycete Meliniomyces variabilis isolated from a sporocarp of Hydnotrya tulasnei (Pezizales) intracellularly colonises roots of ecto-and ericoid mycorrhizal host plants. Czech Mycology 59: 215–226.
- Wang, X.C. & Zhuang, W.Y. (2018) A three-locus phylogeny of Gyromitra (Discinaceae, Pezizales) and discovery of two cryptic species. Mycologia 111: 69–77. https://doi.org/10.1080/00275514.2018.1515456
- Wang, X.C., Yang, Z.L., Chen, S.L., Bau, T., Li, T.H., Li, L., Fan, L. & Zhuang, W.Y. (2023) Phylogeny and taxonomic revision of the family Discinaceae (Pezizales, Ascomycota). Microbiology Spectrum 11: e00207-23. https://doi.org/10.1128/spectrum.00207-23
- White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications 18: 315–322. https://doi.org/10.1016/b978-0-12-372180-8.50042-1
- Xu, A.S. (2000) Two species of Hydnotrya in Xizang. Mycosystem 19: 568–569. https://doi.org/10.3969/j.issn.1672-6472.2000.04.026
- Xu, Y.Y., Wang, Y.W., Li, T., Yan, X.Y. & Fan, L. (2018) DNA analysis reveals rich diversity of Hydnotrya with emphasis on the species found in China. Mycological Progress 17: 1123–1137. https://doi.org/10.1007/s11557-018-1425-2
- Yao, Y.J., Pegler, D.N. & Chase, M.W. (1999) Application of ITS (nrDNA) sequences in the phylogenetic study of Tyromyces s. l. Mycological Research 103: 219–229. https://doi.org/10.1017/s0953756298007138
- Zhang, B.C. (1991) Morphology, cytology and taxonomy of Hydnotrya cerebriformis (Pezizales). Mycotaxon 42: 155–162.