Abstract
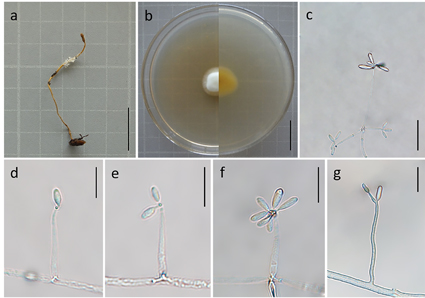
In this study, a new species, Calcarisporium yuanyangense, was isolated from Ophiocordyceps nutans collected from Yuanyang County, Yunnan Province, China, and described by morphology and molecular phylogeny. Based on the combined dataset of five genes including the internal transcribed spacer gene region (ITS), the nuclear ribosomal small and large subunit gene regions (nrSSU and nrLSU), the gene encoding translation elongation factor 1-α (tef-1α), and the RNA polymerase II second largest subunit gene region (rpb2), phylogenetic analyses were performed by the maximum likelihood (ML) and the Bayesian inference (BI) methods to determine the phylogenetic position of C. yuanyangense in Calcarisporium. This species was clustered with C. cordycipiticola, C. xylariicola, and C. arbuscula to form a separate branch. Calcarisporium yuanyangense had the basic morphological characteristics of Calcarisporium, but still differed morphologically from the other species in the genus. This species was similar to C. arbuscula in conidia shape, but the conidia of C. yuanyangense were larger and had a higher length/width ratio than those of C. arbuscula. This study deepens the understanding of the host diversity and the distribution of this taxonomic unit, as well as provides important taxonomic data for further exploration of the genus Calcarisporium.
References
- Barnett, H.L. (1958) A new Calcarisporium parasitic on other fungi. Mycologia 50: 497–500. https://doi.org/10.1080/00275514.1958.12024745
- Barron, G.L. & Peterson, J. (1968) The genera of Hyphomycetes from soil. Soil Science 106 (6): 477. https://doi.org/10.1097/00010694-196812000-00019
- Castlebury, L.A., Rossman, A.Y., Sung, G.H., Hyten, A.S. & Spatafora, J.W. (2004) Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research 108: 864–872. https://doi.org/10.1017/S0953756204000607
- de Hoog, G.S. (1974) The genera Blastobotrys, Sporothrix, Calcarisporium and Calcarisporiella gen. nov. Studies in Mycology 7: 1–83.
- de Hoog, G.S. (1978) Notes on some fungicolous hyphomycetes and their relatives. Persoonia 10: 33–81.
- Deighton, F.C. & Pirozynski, K.A. (1972) Microfungi. V. More hyperparasitic hyphomycetes. Mycological Papers 128: 1–110.
- Evans, H.C. (1971) Thermophilous fungi of coal spoil tips. I. Taxonomy. Transactions of the British Mycological Society 57: 241–254. https://doi.org/10.1016/S0007-1536(71)80006-2
- Haller, B. & Loeffler, W. (1969) Stoffwechselprodukte von Mikroorganismen. Fusidinsaure aus Dermatophyten und anderen Pilzen. Archives of Microbiology 65: 181–194. https://doi.org/10.1007/BF00693320
- Hausner, G. & Reid, J. (2004) The nuclear small subunit ribosomal genes of Sphaeronaemella helvellae, Sphaeronaemella fimicola, Gabarnaudia betae, and Cornuvesica falcata: phylogenetic implications. Canadian Journal of Botany 82 (6): 752–762. https://doi.org/10.1139/b04-046
- Hoang, D.T., Chernomor, O., Haeseler, A.V., Minh, B.Q. & Vinh, L.S. (2018) UFBoot2: Improving the Ultrafast Bootstrap Approximation. Molecular Biology and Evolution 35: 518–522. https://doi.org/10.1093/molbev/msx281
- Hughes, S.J. (1951) Studies on micro-fungi. IX. Calcarisporium, Verticicladium, and Hansfordia (gen. nov.). Mycological Papers 43: 1–25.
- Karlsson, M., Durling, M.B., Choi, J., Kosawang, C., Lackner, G., Tzelepis, G.D., Nygren, K., Dubey, M.K., Kamou, N., Levasseur, A., Zapparata, A., Wang, J., Amby, D.B., Jensen, B., Sarrocco, S., Panteris, E., Lagopodi, A.L., Pöggeler, S., Vannacci, G., Collinge, D.B., Hoffmeister, D., Henrissat, B., Lee, Y.H. & Jensen, D.F. (2015) Insights on the evolution of mycoparasitism from the genome of Clonostachys rosea. Genome Biology and Evolution 7: 465–480. https://doi.org/10.1093/gbe/evu292
- Kirk, P.M., Cannon, P.F., Minter, D.W. & Stalpers, J.A. (2008) Ainsworth and Bisby’s dictionary of the fungi. Commonwealth Mycological Institute. https://doi.org/10.1079/9780851998268.0000
- Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H., Valentin, F., Wallace, I.M., Wilm, A., Lopez, R., Thompson, J.D., Gibson, T.J. & Higgins, D.G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. https://doi.org/10.1093/bioinformatics/btm404
- Li, C.P., Tang, D.X., Wang, Y.B., Fan, Q., Zhang, X.M., Cui, X.L. & Yu, H. (2021) Endogenous bacteria inhabiting the Ophiocordyceps highlandensis during fruiting body development. BMC Microbiology 21: 178. https://doi.org/10.1186/s12866-021-02227-w
- Liu, Q., Xu, Y., Zhang, X., Li, K., Li, X., Wang, F., Xu, F.X. & Dong, C.H. (2021) Infection process and genome assembly provide insights into the pathogenic mechanism of destructive mycoparasite calcarisporium cordycipiticola with host specificity. Journal of Fungi 7 (11): 918. https://doi.org/10.3390/jof7110918
- Liu, Z., Liang, Z.Q., Whalley, A.J.S., Yao, Y.J. & Liu, A.Y. (2001) Cordyceps brittlebankisoides, a new pathogen of grubs and its anamorph, Metarhizium anisopliae var. majus. Journal of Invertebrate Pathology 78: 178–182. https://doi.org/10.1006/jipa.2001.5039
- Maharachchikumbura, S., Hyde, K.D., Jones, E., McKenzie, E., Huang, S.K., Abdel-Wahab, M.A., Daranagama, D.A., Dayarathne, M., D. Souza, M.J., Goonasekara, I.D., Hongsanan, S., Jayawardena, R.S., Kirk, P.M., Konta, S., Liu, J.K., Liu, Z.Y., Norphanphoun, C., Pang, K.L., Perera, R.H., Senanayake, I.C., Shang, Q., Shenoy, B.D., Xiao, Y.P., Bahkali, A.H., Kang, J.C., Somrithipol, S., Suetrong, S., Wen, T.C. & Xu, J.C. (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Diversity 72 (1): 199–301. https://doi.org/10.1007/s13225-015-0331-z
- Maharachchikumbura, S., Hyde, K.D., Jones, E., McKenzie, E., Bhat, J.D., Dayarathne, M.C., Huang, S.K., Xiao, Y.P., D’souza, M.J., Hongsanan, S., Jayawardena, R.S., Daranagama, D.A., Konta, K., Goonasekara, I.D., Zhuang, W.Y., Jeewon, R., Phillips, A., Abdel-Wahab, M.A., Al-Sadi, A.M., Dissanayake, A.J., Kang, J.C., Li, Q.R., Liu, J.K., Liu, X.Z., Liu, Z.Y., Luangsaard, J.J., Pang, K.L., Phookamsak, R., Promputtha, I., Suetrong, S., Stadler, M., Wen, T.C. & Wijayawardene, N.N. (2016) Families of Sordariomycetes. Fungal Diversity 79: 1–317. https://doi.org/10.1007/s13225-016-0369-6
- Matsushima, T. (1975) Icones Microfungorum a Matsushima Lectorum. Published by the Author. Kobe, Japan, pp. 1–209.
- Nguyen, L.T., Schmidt, H.A., von Haeseler, A., Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. https://doi.org/10.1093/molbev/msu300
- Preuss, C.G.T. (1851) Synopsis fungorum hucusque cognitorum praesertim prope Hoyerswerda. Linnaea 24: 101–153.
- Rao, D. & Rao, R. (1964) A new species of Calcarisporium Preuss, from India. Current Science 33: 187–188.
- Rehner, S.A. & Samuels, G.J. (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. https://doi.org/10.1016/S0953-7562(09)80409-7
- Rehner, S.A. & Buckley, E. (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-αsequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97 (1): 84–98. https://doi.org/10.3852/mycologia.97.1.84
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Samuels, G.J., Ismaiel, A., Bon, M.C., De Respinis, S. & Petrini, O. (2010) Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 102: 944–966. https://doi.org/10.3852/09-243
- Sun, J.Z., Dong, C.H., Liu, XZ., Liu, J.K. & Hyde, K.D. (2016) Calcarisporium cordycipiticola sp. nov., an important fungal pathogen of Cordyceps militaris. Phytotaxa 268 (2): 135–144. https://doi.org/10.11646/phytotaxa.268.2.4
- Sun, J.Z., Liu, X.Z., Hyde, K.D., Zhao, Q., Maharachchikumbura, S., Camporesi, E., Bhat, J., Nilthong, S. & Lumyong, S. (2017) Calcarisporium xylariicola sp. nov. and introduction of Calcarisporiaceae fam. nov. in Hypocreales. Mycological Progress 16: 433–445. https://doi.org/10.1007/s11557-017-1290-4
- Sun, J.Z., Liu, X.Z., McKenzie, E., Jeewon, R., Liu, J.K., Zhang, X.L., Zhao, Q. & Hyde, K.D. (2019) Fungicolous fungi: terminology, diversity, distribution, evolution, and species checklist. Fungal Diversity 95: 337–430. https://doi.org/10.1007/s13225-019-00422-9
- Sutton, B.C. (1973) Hyphomycetes from Manitoba and Saskatchewan, Canada. Mycological Papers 132: 1–143.
- Somrithipol, S. & Jones, E.B.G. (2006) Calcarisporium phaeopodium sp. nov., a new hyphomycete from Thailand. Sydowia 58 (1): 133–140.
- Spegazzini, C.L. (1902) Mycetes Argentinenses. Series 2. Anales del Museo Nacionalde Historia Natural de Buenos Aires Series 3 (8): 49–89.
- Swofford, D.L. (2002) PAUP*. Phylogenetic analysis using parsimony (*and other methods), Version 4.0b10. Sinauer Associates, Sunderland, Massachusetts.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. https://doi.org/10.1093/molbev/mst197
- Tubaki, K. (1955) Studies on the Japanese hyphomycetes (II). Fungicolous group. Nagaoa 5: 11–40.
- Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
- Wang, Y.B., Wang, Y., Fan, Q., Duan, D.E., Zhang, G.D., Dai, R.Q., Dai, Y.D., Zeng, W.B., Chen, Z.H., Li, D.D., Tang, D.X., Xu, Z.H., Sun, T., Nguyen, T.T., Tran, N.L., Dao, V.M., Zhang, C.M., Huang, L.D., Liu, Y.J., Zhang, X.M., Yang, D.R., Sanjuan, T., Liu, X.Z., Yang, Z.L. & Yu, H. (2020) Multigene phylogeny of the family Cordycipitaceae (Hypocreales): new taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Diversity 103: 1–46. https://doi.org/10.1007/s13225-020-00457-3
- White, T.J., Bruns, S., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) PCR protocols: a guide to methods and applications. Academic Press Inc., New York, pp. 315–322. https://doi.org/10.1016/b978-0-12-372180-8.50042-1