Abstract
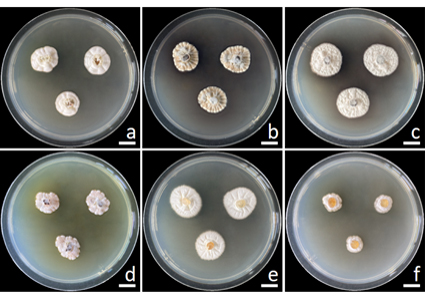
A new species of soil fungi, described herein as Penicillium thailandense, was isolated from soil samples of deciduous dipterocarp forest in Lamphun Province, Thailand. This species was identified by using combination of morphological characteristics and molecular analyses. Morphologically, P. thailandense is similar to P. sanjayi, but it can be distinguished by having monoverticillate and biverticillate conidiophores, white to dark gray sclerotia, and larger conidia. Multi-gene phylogenetic analyses of a combination of the internal transcribed spacer (ITS) of ribosomal DNA, β-tubulin (BenA), calmodulin (CaM), and RNA polymerase II second largest subunit (rpb2) genes revealed that P. thailandense belongs to the Penicillium section Citrina and is distinct from other known species. This paper provides a description, illustrations, and phylogenetic tree of this novel species.
References
- Ashtekar, N., Rajeshkumar, K.C., Yilmaz, N. & Visagie, C.M. (2022) A new Penicillium section Citrina species and series from India. Mycological Progress 21: 42. https://doi.org/10.1007/s11557-022-01802-3
- Barbosa, R.N., Bezerra, J.D.P., da Silva Santos, A.C., Melo, R.F.R., Houbraken, J., Oliveira, N.T. & de Souza-Motta, C.M. (2020) Brazilian tropical dry forest (Caatinga) in the spotlight: an overview of species of Aspergillus, Penicillium and Talaromyces (Eurotiales) and the description of P. vascosobrinhous sp. nov. Acta Botanica Brasilica 34: 409–429. https://doi.org/10.1590/0102-33062019abb0411
- Chanthasena, P., Penkhrue, W., Khowangklang, P., Sritangos, P. & Nantapong, N. (2021) Antimicrobial potential of fungi isolated from soils of dry dipterocarp forest in Northeast Thailand. Chiang Mai Journal of Science 48: 793–807.
- Coutinho, T.C., Ferreira, M.C., Rosa, L.H., de Oliveira, A.M. & de Oliveira Júnior, E.N. (2020) Penicillium citrinum and Penicillium mallochii: New phytopathogens of orange fruit and their control using chitosan. Carbohydrate Polymers 234: 115918. https://doi.org/10.1016/j.carbpol.2020.115918
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. https://doi.org/10.1038/nmeth.2109
- Demjanová, S., Jevinová, P., Pipová, M. & Regecová, I. (2021) Identification of Penicillium verrucosum, Penicillium commune, and Penicillium crustosum isolated from chicken eggs. Processes 9: 53. https://doi.org/10.3390/pr9010053
- Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. https://doi.org/10.1093/nar/gkh340
- Egbuta, M.A., Mwanza, M. & Babalola, O.O. (2017) Health risks associated with exposure to filamentous fungi. International Journal of Environmental Research and Public Health 14: 719. https://doi.org/10.3390/ijerph14070719
- Felsenstein, J. (1985) Confidence intervals on phylogenetics: an approach using bootstrap. Evolution 39: 783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
- Frisvad, J.C. & Samson, R.A. (2004) Polyphasic taxonomy of Penicillium subgenus Penicillium—A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Studies in Mycology 49: 1–174.
- Glass, N.L. & Donaldson, G.C. (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
- Hall, T. (2004) BioEdit. Ibis Therapeutics, Carlsbad, CA, 92008, USA. Available from: http://www.mbio.ncsu.edu/BioEdit/bioedit.html (accessed 15 March 2021)
- Houbraken, J. & Samson, R.A. (2011) Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology 70: 1–51. https://doi.org/10.3114/sim.2011.70.01
- Houbraken, J., Frisvad, J.C. & Samson, R.A. (2011) Taxonomy of Penicillium section Citrina. Studies in Mycology 70: 53–138. https://doi.org/10.3114/sim.2011.70.02
- Houbraken, J., Kocsubé, S., Visagie, C.M., Yilmaz, N., Wang, X.C., Meijer, M., Kraak, B., Hubka, V., Bensch, K., Samson, R.A. & Frisvad, J.C. (2020) Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): an overview of families, genera, subgenera, sections, series and species. Studies in Mycology 95: 5–169. https://doi.org/10.1016/j.simyco.2020.05.002
- Houbraken, J., Visagie, C.M., Meijer, M., Frisvad, J.C., Busby, P.E., Pitt, J.I., Seifert, K.A., Louis-Seize, G., Demirel, R., Yilmaz, N., Jacobs, K., Christensen, M. & Samson, R.A. (2014) A taxonomic and phylogenetic revision of Penicillium section Aspergilloides. Studies in Mycology 78: 373–451. https://doi.org/10.1016/j.simyco.2014.09.002
- Houbraken, J.A.M.P., Frisvad, J.C. & Samson, R.A. (2010) Taxonomy of Penicillium citrinum and related species. Fungal Diversity 44: 117–133. https://doi.org/10.1007/s13225-010-0047-z
- Jeewon, R. & Hyde, K.D. (2016) Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 7: 1669–1677. https://doi.org/10.5943/mycosphere/7/11/4
- Jerushalmi, S., Maymon, M., Dombrovsky, A. & Freeman, S. (2020) Fungal pathogens affecting the production and quality of medical cannabis in Israel. Plants 9: 882. https://doi.org/10.3390/plants9070882
- Khan, I.H. & Javaid, A. (2023) Penicillium citrinum causing postharvest decay on stored garlic cloves in Pakistan. Journal of Plant Pathology 105: 337. https://doi.org/10.1007/s42161-022-01254-4
- Khuna, S., Suwannarach, N., Kumla, J., Frisvad, J.C., Matsui, K., Nuangmek, W. & Lumyong, S. (2021) Growth enhancement of Arabidopsis (Arabidopsis thaliana) and onion (Allium cepa) with inoculation of three newly identified mineral-solubilizing fungi in the genus Aspergillus section Nigri. Frontiers in Microbiology 12: 705896. https://doi.org/10.3389/fmicb.2021.705896
- Korhonen, K. & Hintikka, V. (1980) Simple isolation and inoculation methods for fungal cultures. Karstenia 20: 19–22. https://doi.org/10.29203/ka.1980.192
- Labuda, R., Bacher, M., Rosenau, T., Gasparotto, E., Gratzl, H., Doppler, M., Sulyok, M., Kubátová, A., Berger, H., Cank, K., Raja, H.A., Oberlies, N.H., Schüller, C. & Strauss, J. (2021) Polyphasic approach utilized for the identification of two new toxigenic members of Penicillium section Exilicaulis, P. krskae and P. silybi spp. nov. Journal of Fungi 7: e557. https://doi.org/10.3390/jof7070557
- Liang, L.-J., Jeewon, R., Dhandevi, P., Durairajan, S.S.K., Li, H., Lin, F.-C. & Wang, H.-K. (2021) A novel species of Penicillium with inhibitory effects against Pyricularia oryzae and fungal pathogens inducing citrus diseases. Frontiers in Cellular and Infection Microbiology 10: 604504. https://doi.org/10.3389/fcimb.2020.604504
- Link, J.H.F. (1809) Observationes in ordines plantarum naturales. Dissertatio I. Magazin Der Gesellschaft Naturforschenden Freunde Berlin (in Latin) 3: 3–42.
- Liu, Y.J., Whelen, S. & Hall, B.D. (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. https://doi.org/10.1093/oxfordjournals.molbev.a026092
- Mok, T., Koehler, A.P., Yu, M.Y., Ellis, D.H., Johnson, P.J. & Wickham, N.W. (1997) Fatal Penicillium citrinum pneumonia with pericarditis in a patient with acute leukemia. Journal of Clinical Microbiology 35: 2654–2656. https://doi.org/10.1128/jcm.35.10.2654-2656.1997
- Nguyen, T.T.T., Noh, K.J.K. & Lee, H.B. (2021) New species and eight undescribed species belonging to the families Aspergillaceae and Trichocomaceae in Korea. Mycobiology 49: 534–550. https://doi.org/10.1080/12298093.2021.1997461
- Nilsson, R.H., Taylor, A.F.S., Adams, R.I., Baschien, C., Johan, B.-P., Cangren, P., Coleine, C., Daniel, H.-M., Glassman, S.I., Hirooka, Y., Irinyi, L., Irđënaitë, R., Martin-Sanchez, P.M., Meyer, W., Oh, S.-Y., Sampaio, J.P., Seifert, K.A., Sklenář, F., Stubbe, D., Suh, S.-O., Summerbell, R., Svantesson, S., Unterseher, M., Visagie, C.M., Weiss, M., Woudenberg, J.H.C., Wurzbacher, C., Van den Wyngaert, S., Yilmaz, N., Yurkov, A., Kõljalg, U. & Abarenkov, K. (2018) Taxonomic annotation of public fungal ITS sequences from the built environment ― a report from an April 10‒11, 2017 workshop (Aberdeen, UK). Mycokeys 28: 65–82. https://doi.org/10.3897/mycokeys.28.20887
- Ouhibi, S., Santos, C., Ghali, R., Soares, C., Hedhili, A., Paterson, R. & Lima, N. (2018) Penicillium tunisiense sp. nov., a novel species of Penicillium section Ramosa discovered from Tunisian orchard apples. International Journal of Systematic and Evolutionary Microbiology 68: 3217–3225. https://doi.org/10.1099/ijsem.0.002962
- Paul, N.C., Park, S., Liu, H., Lee, J.G., Han, G.H., Kim, H. & Sang, H. (2021) Fungi associated with postharvest diseases of sweet potato storage roots and in vitro antagonistic assay of Trichoderma harzianum against the diseases. Journal of Fungi 7: 927. https://doi.org/10.3390/jof7110927
- Peterson, S.W. (2000) Phylogenetic analysis of Penicillium species based on ITS and LSU-rDNA nucleotide sequences. In: Samson, R.A. & Pitt, J.I. (Eds.) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Academic Publishers, Amsterdam, the Netherlands, pp. 163–178.
- Peterson, S.W., Vega, F.E., Posada, F. & Nagai, C. (2005) Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia 97: 659–666. https://doi.org/10.1080/15572536.2006.11832796
- Phookamsak, R., Hyde, K.D., Jeewon, R., Bhat, D.J., Jones, E.B.G., Maharachchikumbura, S.S.N., Raspé, O., Karunarathna, S.C., Wanasinghe, D.N., Hongsanan, S., Doilom, M., Tennakoon, D.S., Machado, A.R., Firmino, A.L., Ghosh, A., Karunarathna, A., Mešić, A., Dutta, A.K., Thongbai, B., Devadatha, B., Norphanphoun, C., Senwanna, C., Wei, D., Pem, D., Ackah, F.K., Wang, G.-N., Jiang, H.-B., Madrid, H., Lee, H.B., Goonasekara, I.D., Manawasinghe, I.S., Kušan, I., Cano, J., Gené, J., Li, J., Das, K., Acharya, K., Raj, K.N.A., Latha, K.P.D., Chethana, K.W.T., He, M.-Q., Dueñas, M., Jadan, M., Martín, M.P., Samarakoon, M.C., Dayarathne, M.C., Raza, M., Park, M.S., Telleria, M.T., Chaiwan, N., Matočec, N., de Silva, N.I., Pereira, O.L., Singh, P.N., Manimohan, P., Uniyal, P., Shang, Q.-J., Bhatt, R.P., Perera, R.H., Alvarenga, R.L.M., Nogal-Prata, S., Singh, S.K., Vadthanarat, S., Oh, S.-Y., Huang, S.-K., Rana, S., Konta, S., Paloi, S., Jayasiri, S.C., Jeon, S.J., Mehmood, T., Gibertoni, T.B., Nguyen, T.T.T., Singh, U., Thiyagaraja, V., Sarma, V.V., Dong, W., Yu, X.-D., Lu, Y.-Z., Lim, Y.W., Chen, Y., Tkalčec, Z., Zhang, Z.-F., Luo, Z.-L., Daranagama, D.A., Thambugala, K.M., Tibpromma, S., Camporesi, E., Bulgakov, T.S., Dissanayake, A.J., Senanayake, I.C., Dai, D.Q., Tang, L.-Z., Khan, S., Zhang, H., Promputtha, I., Cai, L., Chomnunti, P., Zhao, R.-L., Lumyong, S., Boonmee, S., Wen, T.-C., Mortimer, P.E. & Xu, J. (2019) Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Diversity 95: 1–273. https://doi.org/10.1007/s13225-019-00421-w
- Pompranee, P. (2009) Study on soil microorganisms from sugarcane field on sugarcane leaves decomposition. Journal of Science Ladkrabang 18: 42–51. [in Thai]
- Qin, S., Wang, J., Liu, S., Tang, X., Liu, Z., Liu, R., Wang, Y., Song, L., Chen, X. & Cernava, T. (2023) First report of green mold disease caused by Penicillium citrinum on Dictyophora rubrovolvata in China. Plant Disease 107: 966. https://doi.org/10.1094/PDIS-10-21-2291-PDN
- Rambaut, A. (2012) FigTree Tree Figure Drawing Tool Version 131, Institute of Evolutionary 623 Biology, University of Edinburgh, Edinburgh, Scotland. Available from: http://tree.bio.ed.ac.uk/software/figtree/ (accessed 29 August 2023)
- Ronquist, F., Teslenko, M., Van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Saif, F.A., Yaseen, S.A., Alameen, A.S., Mane, S.B. & Undre, P.B. (2020) Identification of Penicillium species of fruits using morphology and spectroscopic methods. Journal of Physics: Conference Series 1644: 012019. https://doi.org/10.1088/1742-6596/1644/1/012019
- Serra, R., Peterson, S., CTCOR & Venâncio, A. (2008) Multilocus sequence identification of Penicillium species in cork bark during plank preparation for the manufacture of stoppers. Research in Microbiology 159: 178–186. https://doi.org/10.1016/j.resmic.2007.12.009
- Stamatakis, A. (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. https://doi.org/10.1093/bioinformatics/btl446
- Sun, X., Hu, Y.-H., Wang, J., Fang, C., Li, J., Han, M., Wei, X., Zheng, H., Luo, X., Jia, Y., Gong, M., Xiao, L. & Song, Z. (2021) Efficient and stable metabarcoding sequencing data using a DNBSEQ-G400 sequencer validated by comprehensive community analyses. Gigabyte 2021: 1–15. https://doi.org/10.46471/gigabyte.16
- Suphaphimol, N., Suwannarach, N., Purahong, W., Jaikang, C., Pengpat, K., Semakul, N., Yimklan, S., Jongjitngam, S., Jindasu, S., Thiangtham, S., Chantawannakul, P. & Disayathanoowat, T. (2022) Identification of microorganisms dwelling on the 19th century Lanna mural paintings from northern Thailand using culture-dependent and -independent approaches. Biology 11: 228. https://doi.org/10.3390/biology11020228
- Tamura, K., Stecher, G., Petersen, D., Filipsky, A. & Kumar, S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolutionary 30: 2725–2729. https://doi.org/10.1093/molbev/mst197
- Thom, C. (1910) Cultural studies of species of Penicillium. Bulletin of the Bureau of Animal Industry United States Department of Agriculture 118: 1–109.
- Torres-Garcia, D., Gené, J. & García, D. (2022) New and interesting species of Penicillium (Eurotiomycetes, Aspergillaceae) in freshwater sediments from Spain. MycoKeys 86: 103–145. https://doi.org/10.3897/mycokeys.86.73861
- Větrovský, T., Morais, D., Kohout, P., Lepinay, C., Algora, C., Awokunle Hollá, S., Bahnmann, B.D., Bílohnědá, K., Brabcová, V., D’Alò, F., Human, Z.R., Jomura, M., Kolařík, M., Kvasničková, J., Lladó, S., López-Mondéjar, R., Martinović, T., Mašínová, T., Meszárošová, L., Michalčíková, L., Michalová, T., Mundra, S., Navrátilová, D., Odriozola, I., Piché-Choquette, S., Štursová, M., Švec, K., Tláskal, V., Urbanová, M., Vlk, L., Voříšková, J., Žifčáková, L. & Baldrian, P. (2020) GlobalFungi, a global database of fungal occurrences from high-throughput-sequencing metabarcoding studies. Scientific Data 7: 228. https://doi.org/10.1038/s41597-020-00647-3
- Visagie, C.M. & Yilmaz, N. (2023) Along the footpath of Penicillium discovery: six new species from the Woodville Big Tree Forest Trail. Mycologia 115: 87–106. https://doi.org/10.1080/00275514.2022.2135915
- Visagie, C.M., Houbraken, J., Frisvad, J.C., Hong, S.-B., Klaassen, C.H.W., Perrone, G., Seifert, K.A., Varga, J., Yaguchi, T. & Samson, R.A. (2014b) Identification and nomenclature of the genus Penicillium. Studies in Mycology 78: 343–371. http://dx.doi.org/10.1016/j.simyco.2014.09.001
- Visagie, C.M., Seifert, K.A., Houbraken, J., Samson, R.A. & Jacobs, K. (2014a) Diversity of Penicillium section Citrina within the fynbos biome of South Africa, including a new species from a Protea repens infructescence. Mycologia 106: 537–552. https://doi.org/10.3852/13-256
- Visagie, C.M., Seifert, K.A., Houbraken, J., Samson, R.A. & Jacobs, K. (2016) A phylogenetic revision of Penicillium sect. Exilicaulis, including nine new species from fynbos in South Africa. IMA Fungus 7: 75–117. https://doi.org/10.5598/imafungus.2016.07.01.06
- Vu, D., Groenewald, M., de Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., Al-Hatmi, A., Groenewald, J.Z., Cardinali, G., Houbraken, J., Boekhout, T., Crous, P.W., Robert, V. & Verkley, G.J.M. (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 1–20. https://doi.org/10.1016/j.simyco.2018.05.001
- Wainwright, B.J., Zahn, G.L., Zushi, J., Lee, N.L.Y., Ooi, J.L.S., Lee, J.N. & Huang, D. (2019) Seagrass‐associated fungal communities show distance decay of similarity that has implications for seagrass management and restoration. Ecology and Evolution 9: 11288–11297. https://doi.org/10.1002/ece3.5631
- Wang, X.-C., Chen, K., Zeng, Z.-Q. & Zhuang, W.-Y. (2017) Phylogeny and morphological analyses of Penicillium section Sclerotiora (fungi) lead to the discovery of five new species. Scientific Reports 7: 8233. https://doi.org/10.1038/s41598-017-08697-1
- White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & Whitish, T.J. (Eds.) PCR Protocols, a Guide to Methods and Applications. Academic Press, San Diego, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- Wingfield, L.K., Jitprasitporn, N. & Che-alee, N. (2023) Isolation and characterization of halophilic and halotolerant fungi from man-made solar salterns in Pattani Province, Thailand. PLoS One 18: e0281623. https://doi.org/10.1371/journal.pone.0281623
- Yin, G., Zhang, Y., Pennerman, K.K., Wu, G., Hua, S.S.T., Yu, J., Jurick II, W.M., Guo, A. & Bennett, J.W. (2017) Characterization of blue mold Penicillium species isolated from stored fruits using multiple highly conserved loci. Journal of Fungi 3: e12. https://doi.org/10.3390/jof3010012