Abstract
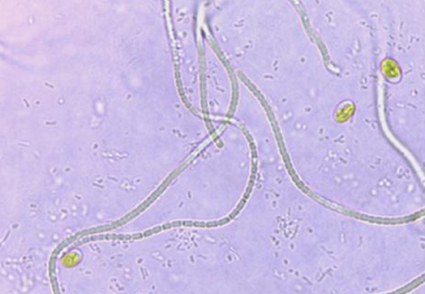
Two novel cyanobacteria (AP3 and AP3b) with thin cells and simple morphology were isolated from two islands of the Indian Sundarbans. The 16S rRNA phylogeny data revealed the distinct lineage of AP3b which was nearest to the clade incorporating the genus Oculatella and Tildeniella. Strain AP3 shared a common ancestor with the species Euryhalinema mangrovii. Additionally, the novel 16S rRNA gene sequences of strains AP3 and AP3b showed similarities about 98% and 93% respectively compared to those of established genera or species to which they were phylogenetically related. Furthermore, the folding patterns of semi-conservative structures like D1-D1’, Box-B and V2 helices of 16S-23S ITS region for both strains AP3 and AP3b displayed significant variations and uniqueness when compared with their respective reference strains (Euryhalinema mangrovii for AP3 and all the genera of Oculatellaceae for AP3b). Strain AP3 shared similar morphological features with its reference strain which confirmed its inter-species relationship. The diagnostic features of AP3b including the presence of necridic cells, aerotopes and a cluster-like growth pattern were found to be very contrasting. Altogether, these results substantiated the establishment of strain AP3b as a novel mono-specific genus named Aerofilum fasciculatum and strain AP3 as the second novel species under the genus Euryhalinema, referred to as Euryhalinema pallustris.
References
<p>Boyer, S.L., Flechtner, V.R. & Johansen, J.R. (2001) Is the 16S–23S rRNA Internal Transcribed Spacer region a good tool for use in molecular systematics and population genetics? A case study in cyanobacteria. <em>Molecular Biology and Evolution </em>18: 1057–1069. https://doi.org/10.1093/oxfordjournals.molbev.a003877</p>
<p>Chakraborty, S., Maruthanayagam, V., Achari, A., Mahansaria, R., Pramanik, A., Jaisankar, P. & Mukherjee, J. (2018) <em>Oxynema aestuarii sp. nov.</em> (Microcoleaceae) isolated from an Indian mangrove forest. <em>Phytotaxa </em>374: 24–40.<em> </em>https://doi.org/10.11646/phytotaxa.374.1.2</p>
<p>Chakraborty, S., Maruthanayagam, V., Achari, A., Pramanik, A., Jaisankar, P. & Mukherjee, J. (2019) <em>Euryhalinema mangrovii gen. nov., sp. nov</em>. and <em>Leptoelongatus litoralis gen. nov., sp. nov.</em> (Leptolyngbyaceae) isolated from an Indian mangrove forest. <em>Phytotaxa </em>422: 58–74. https://doi.org/10.11646/phytotaxa.422.1.4</p>
<p>Dadheech, P.K., Mahmoud, H., Kotut, K. & Krienitz, L. (2012) <em>Haloleptolyngbya alcalis gen. et sp. nov.</em>, a new filamentous cyanobacterium from the soda lake Nakuru, Kenya. <em>Hydrobiologia </em>691: 269–283. https://doi.org/10.1007/s10750-012-1080-6</p>
<p>Debnath, M., Singh, T. & Bhadury, P. (2017) New records of Cyanobacterial morphotypes with <em>Leptolyngbya indica sp. nov.</em> from terrestrial biofilms of the Lower Gangetic Plain, India. <em>Phytotaxa </em>316: 101–120. https://doi.org/10.11646/phytotaxa.316.2.1</p>
<p>Demoulin, C.F., Lara, Y.J., Cornet, L., Francois, C., Baurain, D., Wilmotte, A. & Javaux, E.J. (2019) Cyanobacteria evolution: Insight from the fossil record. <em>Free Radical Biology and Medicine </em>140: 206-223. https://doi.org/10.1016/j.freeradbiomed.2019.05.007</p>
<p>Drummond, A.J., Ho, S.Y.W., Philips, M.J. & Rambaut, A. (2006) Relaxed phylogenetics and dating with confidence. <em>PLoS Biology </em>4: e88. https://doi.org/10.1371/journal.pbio.0040088</p>
<p>Erwin, P.M. & Thacker, R.W. (2008) Cryptic diversity of the symbiotic cyanobacterium <em>Synechococcus spongiarum</em> among sponge host. <em>Molecular Ecology</em> 17: 2937–2947. https://doi.org/10.1111/j.1365-294X.2008.03808.x.</p>
<p>Fiore, M.F., Sant’Anna, C.L., Azevedo, M.T.D., Komarek, J., Kastovsky, J., Sulek, J. & Lorenzi, A.S. (2007) The cyanobacterial genus <em>Brasilonema, gen. nov.</em>, a molecular and phenotypic evaluation. <em>Journal of Phycology </em>43: 789–798. https://doi.org/10.1111/j.1529-8817.2007.00376.x</p>
<p>Fuchsman, C.A., Collins, R.E., Rocap, G. & Brazelton, W.J. (2017) Effect of the environment on horizontal gene transfer between bacteria and archaea. <em>PeerJ </em>5: e3865. https://doi.org/10.7717/peerj.3865</p>
<p>Gelman, A. & Rubin, D.B. (1992) Inference from iterative simulation using multiple sequences. <em>Statistical Science </em>7: 457–472. https://doi.org/10.1214/ss/1177011136</p>
<p>Gonzalez-Resendiz, L., Johansen, J.R., Escobar-Sanchez, V., Segal- Kischinevzky, C., Jimenez-Garcia, L.F. & Leon-Tejera, H. (2018a) Two new species of <em>Phyllonema</em> (Rivulariaceae, Cyanobacteria) with an emendation of the genus. <em>Journal of Phycology</em> 54: 638–652. https://doi.org/10.1111/jpy.12769</p>
<p>Gonzalez-Resendiz, L., Johansen, J.R., Alba-Lois, L., Segal-Kischinevzky, C., Escobar-Sanchez, V., Jimenez Garcia, L.F., Hauer, T. & Leon-Tejera, H. (2018b) <em>Nunduva</em>, a new marine genus of Rivulariaceae (Nostocales, Cyanobacteria) from marine rocky shores. <em>Fottea </em>18: 86–105. https://doi.org/10.5507/fot.2017.018</p>
<p>Gonzalez-Resendiz, L., Johansen, J.R., Leon-Tejera, H., Sanchez, L., Segal-Kischinevzky, C., Escobar-Sanchez, V. & Morales, M. (2019) A bridge too far in naming species: A total evidence approach does not support recognition of four species in<em> Desertifilum</em> (Cyanobacteria). <em>Journal of Phycology </em>55: 898–911. https://doi.org/10.1111/jpy.12867</p>
<p>Iteman, I., Rippka, R., de Marsac, N.T. & Herdman, M. (2000) Comparison of conserved structural and regulatory domains within divergent 16S rRNA–23S rRNA spacer sequences of cyanobacteria. <em>Microbiology </em>146: 1275–1286. https://doi.org/10.1099/00221287-146-6-1275</p>
<p>Johansen, J.R., Kovacik, L., Casamatta, D.A., Fučiková, K. & Kaštovský, J. (2011) Utility of 16S-23S ITS sequence and secondary structure for recognition of intrageneric and intergeneric limits within cyanobacterial taxa: <em>Leptolyngbya corticola sp. nov</em>. (Pseudanabaenaceae, Cyanobacteria). <em>Nova Hedwigia </em>92: 283–302. https://doi.org/10.1127/0029-5035/2011/0092-0283</p>
<p>Johansen, J.R., Mares, J., Pietrasiak, N., Bohunicka, M., Zima, J. Jr, Stenclova, L. & Hauer, T. (2017) Highly divergent 16S rRNA sequences in ribosomal operons of <em>Scytonema hyalinum</em> (Cyanobacteria). <em>PLoS ONE</em> 12: e0186393 https://doi.org/10.1371/journal.pone.0186393</p>
<p>Jung, P., Mikhailyuk, T., Emrich, D., Baumann, K., Dultz, S. & Budel, B. (2020) Shifting boundaries: Ecological and Geographical range extension based on three new species in the cyanobacterial genera <em>Cyanocohniella, Oculatella </em>and<em> Aliterella. Journal of Phycology </em>56 (5): 1216–1231. [Early view] https://doi.org/10.1111/jpy.13025</p>
<p>Komarek, J. & Anagnostidis, K. (2005) <em>Cyanoprokaryota. 2. Teil: Oscillatoriales. In: </em>Büdel, B., Gärdner, G., Krienitz, L. & Schagerl, M. (Eds.) <em>Süsswasserflora von Mitteleuropa</em>. Elsevier, München, 759 pp.</p>
<p>Komarek, J., Kaštovský, J., Ventura, S., Turicchia, S. & Šmarda, J. (2009) The cyanobacterial genus <em>Phormidesmis</em>. <em>Algological Studies </em>129: 41–59. https://doi.org/10.1127/1864-1318/2009/0129-0041</p>
<p>Komarek, J., Kastovsky, J., Mares, J. & Johansen, J.R. (2014) Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) using a polyphasic approach. <em>Preslia </em>86: 295–335.</p>
<p>Lane, D.J. (1991) 16S/23S rRNA sequencing. <em>In: </em>Stackebrandt, E. & Goodfellow, M. (Eds.) <em>Nucleic acid techniques in bacterial systematics. </em>Chichester, United Kingdom: John Wiley and Sons, pp. 115–175.</p>
<p>Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H., Valentin, F., Wallace, I.M., Wilm, A., Lopez, R., Thompson, J.D., Gibson, T.J. & Higgins, D.G. (2007) Clustal W and Clustal X version 2.0. <em>Bioinformatics </em>23: 2947–2948. https://doi.org/10.1093/bioinformatics/btm404</p>
<p>Mai, T., Johansen, J.R., Pietrasiak, N., Bohunicka, M. & Martin, M.P. (2018) Revision of the <em>Synechococcales </em>(Cyanobacteria) through recognition of four families including Oculatellaceae <em>fam. nov</em>. and Trichocoleaceae <em>fam. nov</em>. and six new genera containing 14 species. <em>Phytotaxa </em>365: 1–59. https://doi.org/10.11646/phytotaxa.365.1.1</p>
<p>Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. <em>Proceedings of the Gateway Computing Environments Workshop (GCE)</em> 2010: 1–8. https://doi.org/10.1109/GCE.2010.5676129</p>
<p>Neogi, S.B., Dey, M., Kabir, S.M.L., Masum, S.J.H., Kopprio, G., Yamasaki, S. & Lara, R. (2016) Sundarban mangroves: diversity, ecosystem services and climate change impacts. <em>Asian Journal of Medical and Biological Research </em>2: 488–507. https://doi.org/10.3329/ajmbr.v2i4.30988</p>
<p>Nübel, U., Garcia-Pichel, F. & Muyzer, G. (1997) PCR primers to amplify 16S rRNA genes from cyanobacteria. <em>Applied Environmental Microbiology </em>63: 3327–3332.</p>
<p>Osorio-Santos, K., Pietrasiak, N., Bohunická, M., Miscoe, L.H., Kovacik, L., Martin, M.P. & Johansen, J.R. (2014) Seven new species of <em>Oculatella </em>(Pseudanabaenales, Cyanobacteria) <em>European Journal of Phycology </em>49: 450–470. https://doi.org/10.1080/09670262.2014.976843</p>
<p>Perkerson III, R.B., Johansen, J.R., Kovacik, L., Brand, J., Kastovsky, J. & Casamatta, D.A. (2011) A unique Pseudanabaenalean (cyanobacteria) genus <em>Nodosilinea </em>gen. nov. based on morphological and molecular data. <em>Journal of Phycology </em>47: 1397–1412. https://doi.org/10.1111/j.1529-8817.2011.01077.x</p>
<p>Pietrasiak, N., Muhlsteinova, R., Siegesmund, M.A. & Johansen, J.R. (2014) Phylogenetic placement of <em>Symplocastrum</em> (Phormidiaceae) with a new combination <em>S. californicum</em> and two new species: <em>S. flechtnerae</em> and <em>S. torsivum</em>. <em>Phycologia</em> 53: 529–541. https://doi.org/10.2216/14-029.1</p>
<p>Pramanik, A., Sundararaman, M., Das, S., Ghosh, U. & Mukherjee, J. (2011) Isolation and characterization of cyanobacteria possessing antimicrobial activity from the Sundarbans, the world’s largest tidal mangrove forest. <em>Journal of Phycology </em>47: 731–743. https://doi.org/10.1111/j.1529-8817.2011.01017.x</p>
<p>Řeháková, K., Johansen, J.R., Bowen, M.B., Martin, M.P. & Sheil, C.A. (2014) Variation in secondary structure of the 16S rRNA molecule in cyanobacteria with implications for phylogenetic analysis. <em>Fottea</em> 14: 161–178. https://doi.org/10.5507/fot.2014.013</p>
<p>Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M. & Stanier, R.Y. (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. <em>Journal of General Microbiology</em> 111: 1–61. https://doi.org/10.1099/00221287-111-1-1</p>
<p>Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: effecient Bayesian phylogenetic inference and model choice across a large model space. <em>Systematic Biology</em> 61: 539–542. https://doi.org/10.1093/sysbio/sys029</p>
<p>Shalygin, S., Shalygina, R., Johansen, J.R., Pietrasiak, N., Berrendero Gomez, E., Bohunicka, M., Mares, J. & Sheil, C.A. (2017) <em>Cyanomargarita</em> gen. nov. (Nostocales, Cyanobacteria): convergent evolution resulting in a cryptic genus. <em>Journal of Phycology</em> 53: 762–777. https://doi.org/10.1111/jpy.12542</p>
<p>Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA 6: Molecular Evolutionary Genetic Analysis Version 6.0. <em>Molecular Biology and Evolution </em>30: 2725-2729. https://doi.org/10.1093/molbev/mst197</p>
<p>Tooming-Klunderud, A., Sogge, H., Rounge, T.B., Nederbragt, A.J., Lagesen, K., Glockner, G., Hayes, P.K., Rohrlack, T. & Jakobsen, K.J. (2013) From Green to Red: Horizontal Gene Transfer of the Phycoerythrin Gene cluster between <em>Planktothrix </em>strains. <em>Applied and Environmental Microbiology </em>79: 6803-6812. https://doi.org/10.1128/AEM.01455-13</p>
<p>Turicchia, S., Ventura, S., Komárková, J. & Komárek, J. (2009) Taxonomic evaluation of cyanobacterial microflora from alkaline marshes of northern Belize: 2. Diversity of oscillatorialean genera. <em>Nova Hedwigia </em>89: 65–200. https://doi.org/10.1127/0029-5035/2009/0089-0165</p>
<p>Vazquez-Martinez, J., Gutierrez-Villagomez, J.M., Fonesca-Garcia, C., Ramirez-Chavez, E., Mondragon-Sanchez, M.L., Partida- Martinez, L., Johansen, J.R. & Molina-Torres, J. (2018) <em>Nodosilinea chupicuarensis</em> <em>sp. nov.</em> (Leptolyngbyaceae, Synechococcales) a subaerial cyanobacterium isolated from a stone monument in central Mexico. <em>Phytotaxa</em> 334: 167–182. https://doi.org/10.11646/phytotaxa.334.2.6</p>
<p>Vinogradova, O., Mikhailyuk, T., Glaser, K., Holzinger, A. & Karsten, U. (2017) New species of <em>Oculatella (Synechococcales, Cyanobacteria) </em>from terrestrial habitats of Ukraine. <em>Ukranian Botanical Journal </em>74: 509–520. https://doi.org/10.15407/ukrbotj74.06.509</p>
<p>Yarza, P., Yilmaz, P., Pruesse, E., Glöckner, F.O., Ludwig, W., Schleifer, K.H., Witman, W.B., Euzéby, J., Amann, R. & Rosselló-Móra, R. (2014) Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. <em>Nature Reviews Microbiology </em>12: 635–645. https://doi.org/10.1038/nrmicro3330</p>
<p>Zammit, G. (2018) Systematics and biogeography of sciophilous cyanobacteria; an ecological and molecular description of <em>Albertania skiophila </em>(Leptolyngbyaceae) <em>gen. & sp. nov</em>. <em>Phycologia </em>57: 481–491. https://doi.org/10.2216/17-125.1</p>
<p>Zammit, G., Billi, D. & Albertano, P. (2012) The subaerophytic cyanobacterium <em>Oculatella subterranea </em>(Oscillatoriales, Cyanophyceae) <em>gen. et sp. nov</em>.: a cytomorphological and molecular description. <em>European Journal of Phycology </em>47: 341–354. https://doi.org/10.1080/09670262.2012.717106</p>
<p>Zhou, W., Ding, D., Yang, Q., Ahmad, M., Zhang, Y., Lin, X., Zhang, Y., Ling, J. & Dong, J. (2018) <em>Marileptolyngbya sina gen. nov., sp. nov. </em>and <em>Salileptolyngbya diazotrophicum gen. nov., sp. nov. </em>(Synechococcales, Cyanobacteria), species of cyanobacteria isolated from a marine ecosystem. <em>Phytotaxa </em>383: 75–92. https://doi.org/10.11646/phytotaxa.383.1.4</p>
<p>Zuker, M. (2003) Mfold web server for nucleic acid folding and hybridization prediction. <em>Nucleic Acids Research</em> 31: 3406–3415. https://doi.org/10.1093/nar/gkg595</p>