Abstract
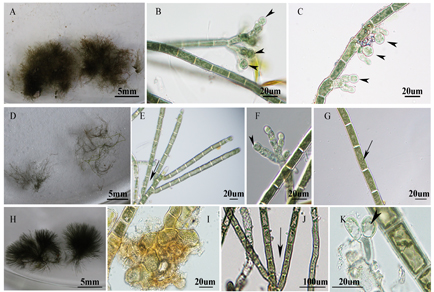
The “Chantransia” is an asexual reproductive stage in the life history of freshwater red algal orders Batrachospermales and Thoreales. Due to the lack of diagnostic features, samples at this stage need to be combined with morphological comparison and molecular phylogenetic analysis to determine their taxonomic affinities. In this study, five bluish “Chantransia” isolates collected from Guangdong and Shanxi Provinces of China were detected as belonging to two species. Among them, isolates HT2001 and HT2103 are the “Chantransia” of Virescentia guangxiensis and have the following features: microscopic thalli, the erect system consisting of filaments with cylindrical cells, undifferentiated into proximal and distal parts, and relatively small monosporangia (< 20 µm in diameter). The three other isolates (GD231, GD233 and GD234), from Dinghu Mountain of Guangdong Province, are characterized by the following features: microscopic thalli with a well-developed prostrate system composed of creeping and loosely aggregated filaments, and the erect system consisting of filaments with cylindrical cells and small monosporangia (< 20 µm in diameter). Phylogenetic analyses based on rbcL and COI-5P supported the proposal of a new species—V. dinghuensis sp. nov. The description of this new species contributes to a more comprehensive understanding of the species diversity and biogeographical distribution of the genus Virescentia in China.
References
- Aristya, G.R., Fontana, S., Pok, W.L., Necchi, O.Jr. & Liu, S.L. (2024) Virescentia asiatica sp. nov. (Batrachospermales Rhodophyta), a new freshwater red alga from East Asia. Cryptogamie, Algologie 45: 77–88. https://doi.org/10.5252/cryptogamie-algologie2024v45a7
- Carmona, J.J., Gustavo, M. & Necchi, O.Jr. (2006) Ecology and morphological characterization of gametophyte and ‘Chantransia’ stages of Sirodotia huillensis (Batrachospermales, Rhodophyta) from a stream in central Mexico. Phycological Research 54: 108–115. https://doi.org/10.1111/j.1440-1835.2006.00417.x
- Chiasson, W.B., Sabo, N.J. & Vis, M.L. (2005) Affinities of freshwater putative chantransia stages (Rhodophyta) from molecular and morphological data. Phycologia 44 (2): 163–168. https://doi.org/10.2216/0031-8884(2005)44[163:AOFPCS]2.0.CO;2
- De Castro Agostinho, D. & Necchi, O.Jr. (2014) Systematics of the section Virescentia of the genus Batrachospermum (Batrachospermales, Rhodophyta) in Brazil. Phycologia 53 (6): 561–570. https://doi.org/10.2216/PH14-034.1
- Entwisle, T.J., Vis, M.L., Chiasson, W.B., Necchi, O.Jr. & Sherwood, A.R. (2009) Systematics of the Batrachospermales (Rhodophyta)—a synthesis. Journal of Phycology 45 (3): 704–715. https://doi.org/10.1111/j.1529-8817.2009.00686.x
- Fang, K.P., Nan, F.R., Feng, J., Lü, J.P., Liu, Q., Liu, X.D. & Xie, S.L. (2021) Virescentia guangxiensis (Batrachospermales, Rhodophyta): a new freshwater red algal species from South China. Journal of Oceanology and Limnology 39: 1538–1546. https://doi.org/10.1007/s00343-020-0225-0
- Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic biology 59: 307–321. https://doi.org/10.1093/sysbio/syq010
- Guiry, M.D. & Guiry, G.M. (2022) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available from: http://www.algaebase.org (accessed 7 June 2025)
- Guo, W.N., Nan, F.R., He, Z.S., Liu, X.D., Liu, Y., Liu, Q., Feng, J. & Xie, S.L. (2025) Morphology and molecular phylogeny of Virescentia guangxiensis (Batrachospermaceae, Rhodophyta). Phytotaxa 687 (1): 84–100. https://doi.org/10.11646/phytotaxa.687.1.6
- Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. https://doi.org/10.1021/bk-1999-0734.ch008
- Hambrook, J.A. & Sheath, R.G. (1991) Reproductive ecology of the freshwater red alga Batrachospermum boryanum Sirodot in a temperate headwater stream. Hydrohiologia 218: 233–246. https://doi.org/10.1007/bf00038837
- Han, J.F., Nan, F.R., Feng, J., Lv, J.P., Liu, Q., Liu, X.D. & Xie, S.L. (2020) Affinities of four freshwater putative “Chantransia” stages (Rhodophyta) in Southern China from molecular and morphological data. Phytotaxa 441 (1): 47–59. https://doi.org/10.11646/phytotaxa.441.1.4
- Han, J.F., Nan, F.R., Feng, J., Lv, J.P., Liu, Q., Liu, X.D. & Xie, S.L. (2021) Affinities of freshwater “Chantransia” stage algae (Rhodophyta) from China based on molecular and morphological analyses. Journal of Oceanology and Limnology 39 (3): 1063–1076. https://doi.org/10.1007/s00343-020-0114-6
- Han, J.F., Nan, F.R., Feng, J., Lv, J.P., Liu, Q., Liu, X.D. & Xie, S.L. (2022) Thorea baiyunensis sp. nov. (Thoreales, Rhodophyta) and T. okadae, a new record from China. PhytoKeys 193: 107–123. https://doi.org/10.3897/phytokeys.193.79667
- Hanyuda, T., Suzawa, Y., Suzawa, T., Arai, S., Sato, H., Ueda, K. & Kumano, S. (2004) Biogeography and taxonomy of Batrachospermum helminthosum (Batrachospermales, Rhodophyta) in Japan inferred from rbcL gene sequences. Journal of Phycology 40 (3): 581–588. https://doi.org/10.1111/j.1529-8817.2004.03159.x
- Higa, A., Kasai, F., Kawachi, M., Kumano, S., Sakayama, H., Miyashita, M. & Watanabe, M.M. (2007) Seasonality of gametophyte occurrence, maturation and fertilization of the freshwater red alga Thorea okadae (Thoreales, Rhodophyta) in the Kikuchi River, Japan. Phycologia 46 (2): 160–167. https://doi.org/10.2216/05-39.1
- Hirose, H. & Seto, R. (1959) Some new knowledge of the chantransia stage of Batrachospertmum moniliforme Roth. Bulletin of the Japanese Society of Phycology 7: 52–55. [in Japanese]
- Ji, L., Xie, S.L., Feng, J., Chen, L. & Wang, J. (2014) Molecular systematics of four endemic Batrachospermaceae (Rhodophyta) species in China with multilocus data. Journal of Systematics and Evolution 52 (1): 92–100. https://doi.org/10.1111/jse.12058
- Johnston, E.T., Dixon, K.R., West, J.A., Buhari, N. & Vis, M.L. (2018) Three gene phylogeny of the Thoreales (Rhodophyta) reveals high species diversity. Journal of Phycology 54 (2): 159–170. https://doi.org/10.1111/jpy.12618
- Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., von Haeseler, A. & Jermiin, L.S. (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. https://doi.org/10.1038/nmeth.4285
- Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010
- Kitayama, T. & Suzuki, M. (2024) Sheathia yedoensis, a New Species of the Freshwater Red Alga (Batrachospermaceae, Rhodophyta) from Kitanomaru Park, Adjacent to the Imperial Palace, Tokyo, Japan. Bulletin of the National Museum of Nature and Science. Series B, Botany 50 (4): 131–140. https://doi.org/10.50826/bnmnsbot.50.4_131
- Kumano, S. (2002) Freshwater Red Algae of the World. Biopress Ltd, Bristol, UK, 375 pp.
- Nan, F.R., Feng, J., Han, X.J., Lv, J.P., Liu, Q. & Xie, S.L. (2016) Molecular identification of Audouinella-like species (Rhodophyta) from China based on three short DNA fragments. Phytotaxa 246 (2): 107–119. https://doi.org/10.11646/phytotaxa.246.2.2
- Necchi, O.Jr., Agostinho, D.C. & Vis, M.L. (2018) Revision of Batrachospermum section Virescentia (Batrachospermales, Rhodophyta) with the establishment of the new genus, Virescentia stat. nov. Cryptogamie Algologie 39 (3): 313–338. https://doi.org/10.7872/crya/v39.iss3.2018.313
- Necchi, O.Jr., García Filho, F.A. & Paiano, M.O. (2019b) Revision of Batrachospermum sections Acarposporophytum and Aristata (Batrachospermales, Rhodophyta) with the establishment of the new genera Acarposporophycos and Visia. Phytotaxa 395 (2): 51–65. https://doi.org/10.11646/phytotaxa.395.2.1
- Necchi, O.Jr. & Oliveira, M.C. (2011) Phylogenetic affinities of “Chantransia” stages in members of the Batrachospermales and Thoreales (Rhodophyta). Journal of Phycology 47 (3): 680–686. https://doi.org/10.1111/j.1529-8817.2011.00997.x
- Necchi, O.Jr., West, J.A., Ganesan, E.K., Yasmin, F., Rai, S.K. & Rossignolo, N.L. (2019a) Diversity of the genus Sheathia (Batrachospermales, Rhodophyta) in northeast India and east Nepal. Algae 34 (4): 277–288. https://doi.org/10.4490/algae.2019.34.10.30
- Necchi, O.Jr. & Zucchi, M.R. (1995) Systematics and distribution of freshwater Audouinella (Acrochaetiaceae, Rhodophyta) in Brazil. European Journal of Phycology 30 (3): 209–218. https://doi.org/10.1080/09670269500650991
- Necchi, O.Jr. & Zucchi, M.R. (1997) Audouinella macrospora (Acrochaetiaceae, Rhodophyta) is the chantransia stage of Batrachospermum (Batrachospermaceae). Phycologia 36 (3): 220–224. https://doi.org/10.2216/i0031-8884-36-3-220.1
- Nguyen, L.T., Schmidt, H.A., Haeseler, A.v. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. https://doi.org/10.1093/molbev/msu300
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Rossignolo, N.L. & Necchi, O.Jr. (2016) Revision of section Setacea of the genus Batrachospermum (Batrachospermales, Rhodophyta) with emphasis on specimens from Brazil. Phycologia 55 (4): 337–346. https://doi.org/10.2216/15-144.1
- Rueness, J. (2010) DNA barcoding of select freshwater and marine red algae (Rhodophyta). Cryptogamie, Algologie 31 (4): 377–386.
- Salomaki, E.D., Kwandrans, J., Eloranta, P. & Vis, M.L. (2014) Molecular and morphological evidence for Sheathia gen. nov. (Batrachospermales, Rhodophyta) and three new species. Journal of Phycology 50 (3): 526–542. https://doi.org/10.11646/phytotaxa.345.2.1
- Saunders, G.W. (2005) Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society B. Biological Sciences 360 (1462): 1879–1888. https://doi.org/10.1098/rstb.2005.1719
- Sheath, R.G. & Hambrook, J.A. (1990) Freshwater ecology. In: Cole, K.M. & Sheath, R.G. (Eds.) Biology of the Red Algae. Cambridge University Press, Cambridge, pp. 433–453.
- Sheath, R.G. & Hytnes, B.J. (1980) A preliminary investigation of the freshwater red algae in streams of southern Ontario, Canada. Canadian Journal of Botany 583: 1295–1318. https://doi.org/10.1139/b80-161
- Shi, Z.X. (2006) Flora algarum sinicarum aquae dulcis, Tomus Xiii, Rhodophyta, Phaeophyta. 1st ed. Beijing Science Press, Beijing, 208 pp.
- Sirodot, S. (1873) Nouvelle classification des algues d’eau douce du genre Batrachospermum: développement; générations alternantes. Comptes-rendus hebdomadaires des séances de l’Académie des Sciences 76: 1216–1120.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. https://doi.org/10.1093/molbev/mst197
- Vis, M.L., Lee, J., Eloranta, P., Chapuis, I.S., Lam, D.W. & Necchi, O.Jr. (2020) Paludicola gen. nov. and Revision of the Species Formerly in Batrachospermum Section Turfosa (Batrachospermales, Rhodophyta). Journal of Phycology 56 (4): 844–861. https://doi.org/10.1111/jpy.13001
- Vis, M.L. & Necchi, O.Jr. (2021) Freshwater Red Algae: Phylogeny, Taxonomy and Biogeography. Springer International Publishing, Cham, 338 pp.
- Vis, M.L., Saunders, G.W., Sheath, R.G., Dunse, K. & Entwisle, T.J. (1998) Phylogeny of the Batrachospermales (Rhodophyta) inferred from rbcL and 18S ribosomal DNA gene sequences. Journal of Phycology 34: 341–350. https://doi.org/10.1046/j.1529-8817.1998.340341.x
- Xie, S.L. (2001) Studies on Batrachospermales (Rhodophyta) from China. Doctoral dissertation, Institute of Hydrobiology, Chinese Academy of Sciences. [in Chinese with English abstract]
- Xie, S.L., Qiu, M.Y., Nan, F.R., Fang, K.P. & Han, J.F. (2020) Batrachospermales (Rhodophyta) of China: a catalogue and bibliography. Nova Hedwigia 110 (1–2): 37–77. https://doi.org/10.1127/nova_hedwigia/2020/0565
- Yoshida, T. (1959) Life-cycle of a species of Batrachospermum found in northern Kyushu, Japan. Japanese Journal of Botany 17: 29–42.
- Zhang, D., Gao, F., Li, W.-X, Jakovlić, I., Zou, H., Zhang, J. & Wang, G.-T. (2020) PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources 20: 348–355. https://doi.org/10.1111/1755-0998.13096