Abstract
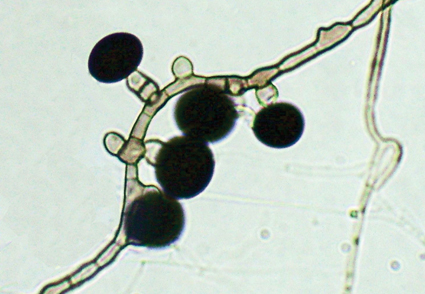
Nigrospora species are found in various substrates as saprobes and are associated with plants as pathogens and endophytes. In this study, we describe N. solani from healthy Solanum lycopersicum var. cerasiforme leaves in an organic farming system in Brazil. Based on morphological data and multi-locus phylogeny using internal transcribed spacer, beta-tubulin, and translation elongation factor 1, N. solani can be recognized as a new species for further research. This anamorphic species is characterized by conidiogenous cells discrete, globose, subglobose, and or ampulliform, solitary, initially hyaline becoming pale brown with age and by conidia solitary, globose to subglobose, black, smooth, and aseptate.
References
- Araújo, W.L., Lima, A.D.S., Azevedo, J.L., Marcon, J., Sobral, J.K. & Lacava, P.T. (2002) Manual: isolamento de microrganismos endofíticos. Piracicaba 1: 86.
- Batista, A.C. & Siqueira, M.W. (1960) Considerações sobre Nigrospora Zimmermann e descrição de N. aerophila n. sp. Univ.
- Bernardi, C., Busso, C., Borin, R.C., Mazaro, S.M. & Saburo, R.S.S. (2021) The First report of Nigrospora sphaerica Associated with Heliocarpus americanus Seeds in Brazil. Floresta e Ambiente 28: 2019010 3. https://doi.org/10.1590/2179-8087-FLORAM-2019-0103
- Brown, K.B., Hyde, K.D. & Guest, D.I. (1998) Preliminary studies on endophytic fungal communities of Musa acuminata species complex in Hong Kong and Australia. Fungal Diversity 1: 27–51.
- Conforto, C., Lima, N.B., Silva, F.J.A., Câmara, M.P.S., Maharachchikumbura, S. & Michereff, S.J. (2019) Characterization of fungal species associated with cladode brown spot on Nopalea cochenillifera in Brazil. European Journal of Plant Pathology 4: 1179–1194. https://doi.org/10.1007/s10658-019-01847-3
- Cosoveanu, A., Hernandez, M., Iacomi-Vasilescu, B., Zhang, X. & Shu, S. (2016) Fungi as endophytes in Chinese Artemisia spp.: juxtaposed elements of phylogeny, diversity and bioactivity. Mycosphere 7: 102–117. https://doi.org/10.5943/mycosphere/7/2/2
- Chen, Q., Bakhshi, M., Balci, Y., Broders, K.D., Cheewangkoon, R., Chen, S.F., Fan, X.L., Gramaje, D., Halleen, F., Jung, M.H., Jiang, N., Jung, T., Májek, T., Marincowitz, S., Milenković, I., Mostert, C.N., Faziha, I.N., Pan, M., Raza, M., Scanu, B., Spies, C.F.J, Suhaizan, L., Suzuki, H., Tian, C.M., Tomšovský, M., Úrbez-Torres, J.R., Wang, W., Wingfield, B.D., Wingfield, M.J., Yang, Q., Yang, X., Zare, R., Zhao, P., Groenewald, J.Z., Cai, L. & Crous, P.W. (2022) Genera of phytopathogenic fungi: GOPHY 4. Studies in Mycology 101: 417. https:// org/10.3114/sim.2022.101.06
- Crous, P.W. & Groenewald, J.Z. (2013) A phylogenetic re-evaluation of Arthrinium. IMA fungus 4: 133–154. https://org/10.5598/imafungus.2013.04.01.13
- Crous, P.W., Carnegie, A.J., Wingfield, M.J., Sharma, R., Mughini, G., Noordeloos, M.E., Santini, A. Shouche, Y.S., Bezerra, J.D.P., Dima, B., Guarnaccia, V., Imrefi, I., Jurjević, Ž., Knapp, D.G., Kovács, G.M., Magistà, D., Perrone, G., Rämä, T., Rebriev, Y.A., Shivas, R.G., Singh, S.M., Souza-Motta, C.M., Thangavel, R., Adhapure, N.N., Alexandrova, A.V., Alfenas, A.C., Alfenas, R.F., Alvarado, P., Alves, A.L., Andrade, D.A., Andrade, J.P., Barbosa, R.N., Barili, A., Barnes, C.W., Baseia, I.G., Bellanger, J.-M., Berlanas, C., Bessette, A.E., Bessette, A.R., Biketova, A.Yu., Bomfim, F.S., Brandrud, T.E., Bransgrove, K., Brito, A.C.Q., Cano-Lira, J.F., Cantillo, T., Cavalcanti, A.D., Cheewangkoon, R., Chikowski, R.S., Conforto, C., Cordeiro, T.R.L., Craine, J.D., Cruz, R., Damm, U., de Oliveira, R.J.V., de Souza, J.T., de Souza, H.G., Dearnaley, J.D.W., Dimitrov, R.A., Dovana, F., Erhard, A., Esteve-Raventós, F., Félix, C.R., Ferisin, G., Fernandes, R.A., Ferreira, R.J., Ferro, L.O., Figueiredo, C.N., Frank, J.L., Freire, K.T.L.S., García, D., Gené, J., Gęsiorska, A., Gibertoni, T.B., Gondra, R.A.G., Gouliamova, D.E., Gramaje, D., Guard, F., Gusmão, L.F.P., Haitook, S., Hirooka, Y., Houbraken, J., Hubka, V., Inamdar, A., Iturriaga, T., Iturrieta-González, I., Jadan, M., Jiang, N., Justo, A., Kachalkin, A.V., Kapitonov, V.I., Karadelev, M., Karakehian, J., Kasuya, T., Kautmanová, I., Kruse, J., Kušan, I., Kuznetsova, T.A., Landell, M.F., Larsson, K.-H., Lee, H.B., Lima, D.X., Lira, C.R.S., Machado, A.R., Madrid, H., Magalhães, O.M.C., Majerova, H., Malysheva, E.F., Mapperson, R.R., Marbach, P.A.S., Martín, M.P., Martín-Sanz, A., Matočec, N., McTaggart, A.R., Mello, J.F., Melo, R.F.R., Mešič, A., Michereff, S.J., Miller, A.N., Minoshima, A., Molinero-Ruiz, L., Morozova, O.V., Mosoh, D., Nabe, M., Naik, R., Nara, K., Nascimento, S.S., Neves, R.P., Olariaga, I., Oliveira, R.L., Oliveira, T.G.L., Ono, T., Ordoñez, M.E., de M. Ottoni, A., Paiva, L.M., Pancorbo, F., Pant, B., Pawłowska, J., Peterson, S.W., Raudabaugh, D.B., Rodríguez-Andrade, E., Rubio, E., Rusevska, K., Santiago, A.L.C.M.A., Santos, A.C.S., Santos, C., Sazanova, N.A., Shah, S., Sharma, J., Silva, B.D.B., Siquier, J.L., Sonawane, M.S., Stchigel, A.M., Svetasheva, T., Tamakeaw, N., Telleria, M.T., Tiago, P.V., Tian, C.M., Tkalčec, Z., Tomashevskaya, M.A., Truong, H.H., Vecherskii, M.V., Visagie, C.M., Vizzini, A., Yilmaz, N., Zmitrovich, I.V., Zvyagina, E.A., Boekhout, T., Kehlet, T., Læssøe, T. & Groenewald, J.Z. (2019) Fungal Planet description sheets: 868–950. Persoonia 42: 291–473. https://doi.org/10.3767/persoonia.2019.42.11
- dos Santos, D.V., da Silva, R.M.F., Aguilera, P., da Silva, G.A., Bezerra, J.L. & Oliveira, R.J.V. (2021) First occurrence of Nigrospora lacticolonia Mei Wang & L. Cai (Xylariales, Ascomycota) in the Neotropical Region. Check List 17: 1243–1248. https://doi.org/10.15560/17.5.1243
- Brito, A.C.Q., de Mello, J.F., de Almeida Souza, A.E., dos Santos Nascimento, S., de Souza-Motta, C.M. & Machado, A.R. (2023) Richness of Nigrospora spp. (Apiosporaceae) in Manihot esculenta in Brazil and the description of three new species. Mycological Progress 22: 37. https://doi.org/10.1007/s11557-023-01887-4
- Gamboa, M.A., Laureano, S. & Bayman, P. (2003) Measuring diversity of endophytic fungi in leaf fragments: does size matter? Mycopathologia 156: 41–45. https://doi.org/10.1023/A:1021362217723
- Glass, N.L. & Donaldson, G.C. (1995) Development of Primer Sets Designed for Use with the PCR to amplify Conserved Genes from Filamentous Ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
- Hao, Y., Aluthmuhandiram, J.V., Chethana, K.T., Manawasinghe, I.S., Li, X., Hyde, K.D., Phillips, A.J.L. & Zhang, W. (2020) Nigrospora species associated with various hosts from Shandong Peninsula, China. Mycobiology 48: 169–183. https://doi.org/10.1080/12298093.2020.1761747
- Hamzah, T.N.T., Lee, S.Y., Hidayat, A., Terhem, R., Faridah-Hanum, I.R. & Mohamed, R. (2018) Diversity and characterization of endophytic fungi isolated from the tropical mangrove species, Rhizophora mucronata, and identification of potential antagonists against the soil-borne fungus, Fusarium solani. Frontiers in microbiology 9: 1707. https://doi.org/10.3389/fmicb.2018.01707
- Huang, D.Y., Nong, X.H., Zhang, Y.Q., Xu, W., Sun, L.Y., Zhang, T. & Han, C.R. (2022) Two new 2, 5—diketopiperazine derivatives from mangrove-derived endophytic fungus Nigrospora camelliae-sinensis S30. Natural Product Research 36: 3651–3656. https://doi.org/10.1080/14786419.2021.1878168
- Katsurayama, A.M., Martins, L.M., Iamanaka, B.T., Fungaro, M.H.P., Silva, J.J., Pitt, J.I. & Taniwaki, M.H. (2020) Fungal communities in rice cultivated in different Brazilian agroclimatic zones: From field to market. Food Microbiology 87: 103378. https://doi.org/10.1016/j.fm.2019.103378
- Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010
- Lee, W., Kim, D.G., Perera, R.H., Kim, J.S., Cho, Y., Lee, J.W. & Lim, Y.W. (2023) Diversity of Nigrospora (Xylariales, Apiosporaceae) species identified in Korean macroalgae including five unrecorded species. Mycobiology 51: 401–409. https://doi.org/10.1080/12298093.2023.2283272
- Liu, S., Wu, J., Wang, H., Lukianova, A., Tokmakova, A., Jin, Z., Tan, S., Chen, S., Wang, Y. Du, Y., Miroshnikov, K.A. & Xie, J. (2022) Soil Layers Impact Lithocarpus Soil Microbial Composition in the Ailao Mountains Subtropical Forest, Yunnan, China. Journal of Fungi 8: 948. https://doi.org/10.3390/jof8090948
- Liu, R., Li, D., Zhang, Z., Liu, S., Liu, X., Wang, Y. & Wang, Y. (2023) Morphological and phylogenetic analyses reveal two new species and a new record of Apiospora (Amphisphaeriales, Apiosporaceae) in China. MycoKeys 95: 27. https://10.3897/mycokeys.95.96400
- Liu, Y., An, J., Safdar, A., Shen, Y., Sun, Y., Shu, W., Tan, X., Zhu, B., Xiao, J., Schirawski, J., He, F. & Zhu, G. (2024) Identification and Characterization of Nigrospora Species and a Novel Species, Nigrospora anhuiensis, Causing Black Leaf Spot on Rice and Wild Rice in the Anhui Province of China. Journal of Fungi 10: 156. https://doi.org/10.3390/jof10020156
- Luo, X., Xi, Y., Shen, C., Wan, M. & Wang, H. (2022) Occurrence of Nigrospora sphaerica causing leaf blight on Chrysanthemum morifolium in China. Crop Protection 157: 105982. https://doi.org/10.1016/j.cropro.2022.105982
- Lu, L., Karunarathna, S.C., Hyde, K.D., Suwannarach, N., Elgorban, A.A.M., Stephenson, S.L. & Tibpromma, S.A.M. (2022) Endophytic fungi associated with coffee leaves in China exhibited in vitro antagonism against fungal and bacterial pathogens. Journal of Fungi 8 (7): 698. https://doi.org/10.3390/jof8070698
- Jiang, N., Voglmayr, H., Ma, C.Y., Xue, H., Piao, C.G. & Li, Y. (2022) A new Arthrinium-like genus of Amphisphaeriales in China. MycoKeys 92: 27–43. https://doi.org/10.3897/mycokeys.92.86521
- Jia, W., Luo, M., Wei, T., Zhang, H., Zeng, Y. & Jiang, Y. (2024) Nigrospora musae and N. oryzae as new causal agents of broad bean leaf spot disease in China. Crop Protection 177: 106567. https://doi.org/10.1016/j.cropro.2023.106567
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: 2010 gateway computing environments workshop GCE. IEEE, pp. 1–8. https://doi.org/10.1109/GCE.2010.5676129
- Moraes, A.M.L., Junqueira, A.C.V., Costa, G.L., Celano, V., Oliveira, P.C. & Coura, J.R. (2001) Fungal flora of the digestive tract of 5 species of triatomines vectors of Trypanosoma cruzi, Chagas 1909. Mycopathologia 151: 41–48. https://doi.org/10.1023/A:1010905420001
- Nylander, J.A., Ronquist, F., Huelsenbeck, J.P., Nieves-Aldrey, J. & Nieves-Aldrey, J. (2004) Bayesian phylogenetic analysis of combined data. Systematic biology 53: 47–67. https://doi.org/10.1080/10635150490264699
- Ola, A.R.B., Lapailaka, T., Wogo, H.E., Henuk, J.B.D., Simamora, A., Mukkun, L., Proksch, P. & Pham, C.D. (2021) Bioactive Secondary Metabolites from the Mangrove Endophytic Fungi Nigrospora oryzae. Indonesian Journal of Chemistry 21: 1016 –1022. https://doi.org/10.22146/ijc.63129
- Oliveira, R.J.V., Bezerra, J.L., Lima, T.E.F., da Silva, G.A., Cavalcanti, M.A.D.Q. (2016) Phaeosphaeria nodulispora, a new endophytic coelomycete isolated from tropical palm (Cocos nucifera) in Brazil. Nova Hedwigia 103: 185–192. https://doi.org/10.1127/nova_hedwigia/2016/0343
- Oliveira, R.J.V., Souza, R.G., Lima, T.E.F. & Cavalcanti, M.A.Q. (2014) Endophytic fungal diversity in coffee leaves (Coffea arabica) cultivated using organic and conventional crop management systems. Mycosphere 5: 523–530. https://doi.org/10.5943/mycosphere/5/4/4
- Oliveira, R.J.V., Sousa, N.M.F., Pinto, N.W.P., Bezerra, J.L., Silva, G.A. & Cavalcanti, M.A.Q. (2020) Seasonality affects the community of endophytic fungi in coconut (Cocos nucifera) crop leaves. Acta Botanica Brasilica 34: 704–711. https://doi.org/10.1590/0102-33062020abb0106
- Pintos, Á. & Alvarado, P. (2021) Phylogenetic delimitation of Apiospora and Arthrinium. Fungal Systematics and Evolution 7: 197–221. https://doi.org/10.3114/fuse.2021.07.10
- Rashmi, M., Kushveer, J. & Sarma, V. (2019) A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 10: 798–1079. https://doi.org/10.5943/mycosphere/10/1/19
- Raza, M., Zhang, Z.F., Hyde, K.D., Diao, Y.Z. & Cai, L. (2019) Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal diversity 99: 1–104. https://doi.org/10.1007/s13225-019-00434-5
- Rathod, D.P., Dar, M.A., Gade, A.K., Rai, M.K. & Baba, G. (2014) Griseofulvin producing endophytic Nigrospora oryzae from Indian Emblica officinalis Gaertn: a new report. Austin Journal of Biotechnology & Bioengineeringis 1: 1–5
- Raviraja, N.S., Maria, G.L. & Sridhar, K.R. (2006) Antimicrobial evaluation of endophytic fungi inhabiting medicinal plants of the Western Ghats of India. Engineering in Life Sciences 6: 515–520. https://doi.org/10.1002/elsc.200620145
- Rayner, R.W. (1970) A mycological colour chart. CMI and British Mycological Society, Kew, Surrey, UK.
- Rehner, S. (2001) Primers for Elongation Factor 1-α (EF1-α). Insect Biocontrol Laboratory USDA, ARS, PSI: Beltsville, MD, USA, 2001. pp. 4.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liang, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61 (3): 539–542. https://doi.org/10.1093/sysbio/sys029
- Santos, C.D., da Silva, R.O., Candeias, E.L., Vitória, N.S., Luz, E.D.M. & Bezerra, J.L. (2018) Diversidade de fungos em espécies nativas e cultivadas de orquídeas no Sul da Bahia. Agrotrópica 30: 101–108.
- Santos, D.V., Oliveira, T.G.L., Silva, R.M.F., Silva, G.A., Souza-Motta, C.M., Bezerra, J.L. & Oliveira, R.J.V. (2024) Neoarthrinium
- brasiliense, sp. nov. (Apiosporaceae, Sordariomycetes), a new endophytic fungal species from Lafoensia pacari tree. Sydowia 76:
- –185. https://doi.org/10.12905/0380.sydowia76-2024-0179
- Silva, R.M.F., Neto, W.P., Oliveira, R.J., Bezerra, J.D., Bezerra, J.L., Lima, V.X., Vieira, L.C., Tabosa, J.N., Souza-Motta, C.M. & Silva, G.A. (2023) Effect of climate and phenological stage on fungal endophytes community in Sorghum bicolor leaves. Mycological Progress 22: 19. https://doi.org/10.1007/s11557-023-01870-z
- De Silva, N.I., Maharachchikumbura, S.S.N., Thambugala, K.M., Bhat, D.J., Karunarathna, S.C., Tennakoon, D.S., Phookamsak, R., Jayawardena, R.S., Lumyong, S. & Hyde, K.D. (2021) Morpho-molecular taxonomic studies reveal a high number of endophytic fungi from Magnolia candolli and M. garrettii in China and Thailand. Mycosphere 12: 163–237. https://doi.org/10.5943/mycosphere/12/1/3
- Safi, A., Mehrabi-Koushki, M. & Arzanlou, M. (2024) Endophytic species of Nigrospora from grasses and shrubs of Shadegan International Wetland, with new species and records from Iran. Antonie van Leeuwenhoek 117: 1–16. https://doi.org/10.1007/s10482-024-01976-8
- Senanayake, I.C., Rossi, W., Leonardi, M., Senanayake, I.C., Rossi, W., Leonardi, M., Weir, A., McHugh, M., Rajeshkumar, K.C., Verma, R.K., Karunarathna, S.C., Tibpromma, S., Ashtekar, N., Ashtamoorthy, S.K., Raveendran, S., Kour, G., Singh, A., Peña-Lastra, S., Mateos, A., Kolařík, M., Antonín, V., Ševčíková, H., Esteve, R. Larsson, E., Pancorbo, F., Moreno, G., Altés, A., Turégano, Y., Du, T. Lu, L., Li, Q., Kang, J., Gunaseelan, S., Kezo, K., Kaliyaperumal, M., Fu, J., Samarakoon, M.C., Gafforov, Y., Teshaboeva, S., Kunjan, P.C., Chamaparambath, A., Etayo, J., Rodriguez-Flak, P., Zhurbenko, M.P., Silva, N.I., Tennakoon, D.S., Latha, K.P.D., Manimohan, P., Calabon, M.S., Ahmadpour, A., Heidarian, Z., Alavi, Z., Alavi, F., Ghosta, Y., Azizi, R., Luo, M., Zhao, M., Kularathnage, N.D. , Hua, L., Yang, Y., Liao, C., Zhao, H., Lestari, A.S., Jayasiri, S.C., Yu, F., Lei, L., Liu, J., Karimi, O., Tang, S., Sun, Y., Wang, Y., Zeng, M. , Htet, Z.H., Linaldeddu, B.T., Artur, A., Phillips, A.J.L., Bregan, C., Montecchio, L., Kesel, A., Hustad, V.P., Miller, A.N., Fedosova, A.G. , Kučera, V., Raza, M., Hussain, M., Chen, Y., Thiyagaraja, V., Gomdola, D., Rathnayaka, A.R., Dissanayake, A.J., Suwannarach, N., Hongsanan, S., Maharachchikumbura, S.S.N., Dissanayake, L.S., Wijayawardene, N.N., Phookamsak, R., Lumyong, S., Jones, E.B.G., Yapa, N., Wanasinghe, D.N., Xie, N., Doilom, M., Manawasinghe, I.S., Liu, J., Zhao, Q., Xu, B., Hyde, K.D. & Song, J. (2023) Fungal diversity notes 1611–1716: taxonomic and phylogenetic contributions on fungal genera and species emphasis in south China. Fungal Diversity 122: 161–403 https://doi.org/10.1007/s13225-023-00523-6
- Stamatakis, A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Sun, X., Guo, L.D. & Hyde, K.D. (2011) Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Diversity 47: 85–95. https://doi.org/10.1007/s13225-010-0086-5
- Tan, Y.P. & Shivas, R.G. (2024) Index of Australian Fungi no. 29. Zenodo. https://doi.org/10.5281/zenodo.10602835
- Tan, Y.P. & Shivas, R.G. (2023) Index of Australian Fungi no. 17. Zenodo. https://doi.org/10.5281/zenodo.8401232
- Tennakoon, D.S. Kuo, C.H., Maharachchikumbura, S.S., Thambugala, K.M., Gentekaki, E., Gentekaki, E., Phillips, A.J.L., Bhat, D.J., Wanasinghe, D.N., Silva, N.I., Promputtha, I. & Hyde, K.D. (2021) Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi. Fungal Diversity 108: 1–215. https://doi.org/10.1007/s13225-021-00474-w
- Wang, M., Liu, F., Crous, P.W. & Cai, L. (2017) Phylogenetic reassessment of Nigrospora: ubiquitous endophytes, plant and human pathogens. Persoonia 39: 118–142. https://doi.org/10.3767/persoonia.2017.39.06
- Wang, H., Sang, Z., Chen, Y., Wei, S., Qiu, K., Liu, Z., Zhang, J. & Tan, H. (2022) The chemical constituents of endophytic fungus Nigrospora chinensis of Gannan navel orange. Natural Product Research 38 (3): 530–538. https://doi.org/10.1080/14786419.2022.2125969
- de Wit, P.J. (2024) The tomato crop. In: Agrios’ Plant Pathology. Academic Press, pp. 795–801. https://doi.org/10.1016/B978-0-12-822429-8.00038-8
- Wu, S.H., Chen, Y.W., Shao, S.C., Wang, L.D., Yu, Y., Li, Z.Y., Yang, L. & Li, S.L. (2009) Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. YB-141 of Azadirachta indica. Chemistry & Biodiversity 6: 79–85. https://doi.org/10.1002/cbdv.200700421
- White, T.J., Bruns, T., Lee, S.J.W.T. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications. Academic Press, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- Zhang, Z.F., Zhou, S.Y., Eurwilaichitr, L., Ingsriswang, S., Raza, M., Chen, Q., Zhao, P., Fang, L. & Cai, L. (2021) Culturable mycobiota from Karst caves in China II, with descriptions of 33 new species. Fungal Diversity 106: 29–136. https://doi.org/10.1007/s13225-020-00453-7
- Zhang, Y.Y., Zhang, T., Li, H.Y., Zheng, R., Ren, J., Yang, Q. & Jiang, N. (2024) Nigrospora humicola (Apiosporaceae, Amphisphaeriales), a New Fungus from Soil in China. Diversity 16: 118. https://doi.org/10.3390/d16020118
- Szabo, K., Diaconeasa, Z., Cătoi, A.F. & Vodnar, D.C. (2019) Screening of ten tomato varieties processing waste for bioactive components and their related antioxidant and antimicrobial activities. Antioxidants 8: 292. https://doi.org/10.3390/antiox8080292