Abstract
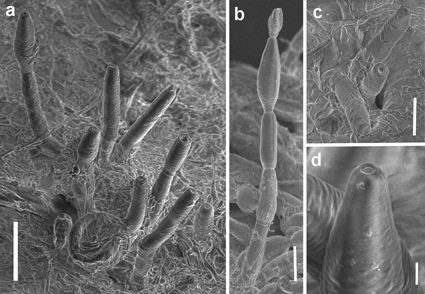
Corynesporopsis ponciri sp. nov. is described from dead twigs, branches, and thorns still attached to trees of Poncirus trifoliata, the trifoliate orange, in Texas, U.S.A. The fungus is characterized by black, punctiform at first, later caespitose and hairy colonies forming large superficial patches covering almost entirely the surface of colonized parts of the host, unbranched conidiophores, solitary or fasciculate and arising from substomatal, compact stromata in loose to dense fascicles and bearing monotretic, mostly determinate, rarely percurrent conidiogenous cells. Conidia are ellipsoidal or fusiform to naviculiform, smooth or verruculose, brown, often with the apical cell light brown, euseptate, with 2, rarely 1 or 3 septa, usually with an associated dark band and forming short, unbranched, acropetal chains of up to four conidia. Multilocus phylogenetic analyses suggested that C. ponciri belongs within the Xylariales and forms a strongly supported monophyletic lineage sister to members of Vamsapriya in Vamsapriyaceae. Corynesporopsis is revealed as polyphyletic based on this and the other two species with available molecular data, C. iberica and C. acaciae. The newly described fungus is compared with morphologically similar species of Corynesporopsis and Heteroconium having 1- or 2-septate conidia and phylogenetic affinities of these polyphyletic anamorphic genera are discussed. The mode and successful colonization of dead parts of the host with conidiophores arising from substomatal stromata suggest that C. ponciri may have a putative endophytic stage and it is capable of switching to a saprobic lifestyle upon senescence or death of the host.
References
- Borowska, A. (1975) New species of Bactrodesmium, Corynespora, Septonema and Taeniolella. Acta Mycologica 11: 59–65. https://doi.org/10.5586/am.1975.005
- Castañeda, R.F., Saikawa, M. & Guarro, J. (1999) A new species of Heteroconium from a tropical rainforest. Mycotaxon 71: 295–300.
- Castañeda, R.F., Silvera, C., Gené, J., Guarro, J., Minter, D.M., Stadler, M. & Saikawa, M. (2010) A new species of Corynesporopsis from Portugal. Mycotaxon 114: 407–415. https://doi.org/10.5248/114.407
- Cao, J.L., He, W.X., Zou, Y.N. & Wu, Q.S. (2023) An endophytic fungus, Piriformospora indica, enhances drought tolerance of trifoliate orange by modulating the antioxidant defense system and composition of fatty acids. Tree Physiology 43: 452–466. https://doi.org/10.1093/treephys/tpac126
- Cheewangkoon, R., Groenewald, J.Z., Hyde, K.D., To-anun, C. & Crous, P.W. (2012) Chocolate spot disease of Eucalyptus. Mycological Progress 11: 61–69. https://doi.org/10.1007/s11557-010-0728-8
- Delgado, G. & Koukol, O. (2016) Microfungi from Nicaragua in a historical collection kept at the Herbarium of the Charles University in Prague. Cryptogamie, Mycologie 37: 15–36. https://doi.org/10.7872/crym/v37.iss1.2016.15
- Delgado, G., Miller, A.N., Hashimoto, A., Iida, T., Ohkuma, M. & Okada, G. (2022) A phylogenetic assessment of Endocalyx (Cainiaceae, Xylariales) with E. grossus comb. et stat. nov. Mycological Progress 21: 221–242. https://doi.org/10.1007/s11557-021-01759-9
- Douanla-Meli, C., Langer, E. & Talontsi, M.F. (2013) Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon. Fungal Ecology 6: 212–222. https://doi.org/10.1016/j.funeco.2013.01.004
- EPPO (2023) EPPO Global Database. Available from: https://gd.eppo.int/taxon/ELSIFA (accessed: 7 March 2023).
- Farr, D.F. & Rossman, A.Y. (2023) Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available from: https://nt.ars-grin.gov/fungaldatabases/ (accessed: 21 March 2023)
- Fawcett, H.S., Lee, H.A. & Piper, C.V. (2012) Citrus Diseases and Their Control. Literary Licensing, LLC, 622 pp.
- HerbIMI (2023) HerbIMI Databases. Available from: http://www.herbimi.info/herbimi/home.htm (accessed: 21 March 2023).
- Hernández, M., Decock, C.A., Costa, M.M. & Crous, P.W. (2022) Phylogeny and taxonomy of Circinotrichum, Gyrothrix, Vermiculariopsiella and other setose hyphomycetes. Persoonia 49: 99–135. https://doi.org/10.3767/persoonia.2022.49.03
- Hernández, M., Gené, J., Castañeda, R.F., Kirk, P.M. & Guarro, J. (2014) A new species of Corynesporopsis from Spain. Mycotaxon 127: 155–160. https://doi.org/10.5248/127.15
- Holubová-Jechová, V. (1987) Studies on hyphomycetes from Cuba VI. New and rare species with tretic and phialidic conidiogenous cells. Česká Mykologie 41: 107–114.
- Index Fungorum (2023) Index Fungorum. Available from: https://www.indexfungorum.org/Names/Names.asp (accessed: 31 January 2023).
- Katoh, K. & Standley, D.M. (2013) MAFFT Multiple Sequence Alignment Software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010
- Kirk, P.M. (1981) New or interesting microfungi II. Dematiaceous Hyphomycetes from Esher Common, Surrey. Transactions of the British Mycological Society 77: 279–297. https://doi.org/10.1016/S0007-1536(81)80031-9
- Kirschner, R. (2015) Phylogenetic placement of a new species of Corynesporopsis from dead acacia wood indicates occurrence of tretic conidiogenesis within Xylariales. Phytotaxa 192: 24–34. https://doi.org/10.11646/phytotaxa.192.1.3
- Koukol, O., Delgado, G., Hofmann, T.A. & Piepenbring, M. (2018) Panama, a hot spot for Hermatomyces (Hermatomycetaceae, Pleosporales) with five new species, and a critical synopsis of the genus. IMA Fungus 9: 107–141. https://doi.org/10.5598/imafungus.2018.09.01.08
- Liu, Y.J., Whelen, S. & Hall, B.D. (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. https://doi.org/10.1093/oxfordjournals.molbev.a026092
- Ma, J., Ma, L.G., Zhang, Y.D., Castañeda, R.F. & Zhang, X.G. (2012a) New species and record of Corynesporopsis and Hemicorynespora from southern China. Nova Hedwigia 95: 233–241. https://doi.org/10.1127/0029-5035/2012/0030
- Ma, L.G., Ma, J., Zhang, Y.D., Castañeda, R.F. & Zhang, X.G. (2012b) New species and records of Heteroconium (anamorphic fungi) from southern China. Mycoscience 53: 466–470. https://doi.org/10.1007/S10267-012-0190-3
- Matsushima, T. (1983) Matsushima Mycological Memoirs No. 3. Published by the author, Kobe.
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE). 14 Nov. 2010, New Orleans, pp. 1–8. https://doi.org/10.1109/GCE.2010.5676129
- Missouri Botanical Garden (2023) Plant Finder Database. Available from: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderSearch.aspx (accessed: 21 February 2023).
- Morgan-Jones, G. (1975) Notes on hyphomycetes XIV. The genus Heteroconium. Mycotaxon 4: 498–503.
- Morgan-Jones, G. (1988) Notes on hyphomycetes. LVII. Corynespora biseptata, reclassified in Corynesporopsis. Mycotaxon 31: 511–515.
- Nicoletti, R. (2019) Endophytic Fungi of Citrus Plants. Agriculture 9: 247. https://doi.org/10.3390/agriculture9120247
- North Carolina Invasive Plant Council (2023) Trifoliate Orange (Hardy Orange or Flying Dragon) Poncirus trifoliata Fact Sheet. Available from: http://nc-ipc.weebly.com/poncirus-trifoliata.html. (accessed: 21 February 2023).
- O’Donnell, K. (1993) Fusarium and its near relatives. In: Reynolds, D.R. & Taylor, J.W. (eds.) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematic. CAB International, Wallingford, pp. 225–233.
- Parfitt, D., Hunt, J., Dockrell, D., Rogers, H.J. & Boddy, L. (2010) Do all trees carry the seeds of their own destruction? PCR reveals numerous wood decay fungi latently present in sapwood of a wide range of angiosperm trees. Fungal Ecology 3: 338–346. https://doi.org/10.1016/j.funeco.2010.02.001
- Peng, Z., Bredeson, J.V., Wu, G.A., Shu, S., Rawat, N., Du, D., Parajuli, S., Yu, Q., You, Q., Rokhsar, D.S., Gmitter, F.G., Jr & Deng, Z. (2020) A chromosome-scale reference genome of trifoliate orange (Poncirus trifoliata) provides insights into disease resistance, cold tolerance and genome evolution in Citrus. The Plant Journal 104: 1215–1232. https://doi.org/10.1111/tpj.14993
- Rambaut, A. (2009) FigTree v1.4: Tree Figure Drawing Tool. Available from: http://tree.bio.ed.ac.uk/software/figtree/ (first accessed: 4 February 2023).
- Rashmi, M., Kushveer, J.S. & Sarma, V.V. (2019) A worldwide list of endophytic fungi with notes on ecology and diversity. Mycosphere 10: 798–1079. https://doi.org/10.5943/mycosphere/10/1/19
- Ronquist, F. & Huelsenbeck, J.P. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
- Samarakoon, M.C., Hyde, K.D., Maharachchikumbura, S.S.N., Stadler, J., Gareth Jones, E.B., Promputtha, I., Suwannarach, N., Camporesi, E., Bulgakov, T.S. & Liu, J.K. (2022) Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Diversity 112: 1–88. https://doi.org/10.1007/s13225-021-00495-5
- Stamatakis, A. (2014) RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. https://doi.org/10.1093/molbev/mst197
- Texas Invasive Species Institute (2023) Texas Invasive Species Institute. Available from: http://www.tsusinvasives.org/home/ (accessed: 21 February 2023).
- Timmer, L.W., Garnsey, S.M. & Graham, J.H. (2000) Compendium of citrus diseases. 2nd Edition. APS Press, St. Paul. https://doi.org/10.1094/9780890545850
- Sun, Y.R., Liu, N.G., Samarakoon, M.C., Jayawardena, R.S., Hyde, K.D. & Wang, Y. (2021) Morphology and phylogeny reveal Vamsapriyaceae fam. nov. (Xylariales, Sordariomycetes) with two novel Vamsapriya species. Journal of Fungi 7: 891. https://doi.org/10.3390/jof7110891
- Varghese, K.I.M. & Rao, V.G. (1980) An undescribed species of Heteroconium Petr. (Hyphomycete) from south India. Current Science 49: 359.
- White, T.J., Bruns, T., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- Yuan, H.-S., Lu, X., Dai, Y.-C., Hyde, K.D., Kan, Y.-H., Kušan, I., He, S.-H., Liu, N.-G., Sarma, V.V., Zhao, C.-L., Cui, B.-K., Yousaf, N., Sun, G., Liu, S.-Y., Wu, F., Lin, C.-G., Dayarathne, M.C., Gibertoni, T.B., Conceição, L.B., Garibay-Orijel, R., Villegas-Ríos, M., Salas-Lizana, R., Wei, T.-Z., Qiu, J.-Z., Yu, Z.-F., Phookamsak, R., Zeng, M., Paloi, S., Bao, D.-F., Abeywickrama, P.D., Wei, D.-P., Yang, J., Manawasinghe, I.S., Harishchandra, D., Brahmanage, R.S., de Silva, N.I., Tennakoon, D.S., Karunarathna, A., Gafforov, Y., Pem, D., Zhang, S.-N., de Azevedo Santiago, A.L.C.M., Bezerra, J.D.P., Dima, B., Acharya, K., Alvarez-Manjarrez, J., Bahkali, A.H., Bhatt, V.K., Brandrud, T.E., Bulgakov, T.S., Camporesi, E., Cao, T., Chen, Y.-X., Chen, Y.-Y., Devadatha, B., Elgorban, A.M., Fan, L.-F., Du, X., Gao, L., Gonçalves, C.M., Gusmão, L.F.P., Huanraluek, N., Jadan, M., Jayawardena, R.S., Khalid, A.N., Langer, E., Lima, D.X., de Lima-Júnior, N.C., de Lira, C.R.S., Liu, J.-K. (Jack), Liu, S., Lumyong, S., Luo, Z.-L., Matočec, N., Niranjan, M., Oliveira-Filho, J.R.C., Papp, V., Pérez-Pazos, E., Phillips, A.J.L., Qiu, P.-L., Ren, Y., Castañeda Ruiz, R.F., Semwal, K.C., Soop, K., de Souza, C.A.F., Souza-Motta, C.M., Sun, L.-H., Xie, M.-L., Yao, Y.-J., Zhao, Q. & Zhou, L.-W. (2020) Fungal diversity notes 1277–1386: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 104: 1–266. https://doi.org/10.1007/s13225-020-00461-7