Abstract
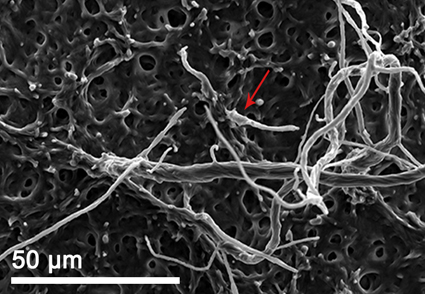
Serendipita is a prominent fungal genus associated with orchids. In this study, a new species of orchid mycorrhizal fungus, Serendipita officinale, is described and thoroughly studied using morphological and molecular approaches. The fungus was isolated from naturally occurring protocorms of Dendrobium officinale in Chongqing Province, China. S. officinale has been observed to actively promote the germination of seeds and facilitate the seedling development of D. officinale, forming typical mycorrhizal pelotons within protocorm cells. This species is characterized by its characteristics on PDA medium, including the presence of abundant villiform and felty aerial mycelium, uninucleate hyphal cells, and the highest growth rate in terms of colony diameter (78 mm diam. in 2 weeks). Furthermore, analyses of ITS rDNA and LSU sequences also provide additional support for the novelty of this new plant symbiont.
References
- Basiewicz, M., Weiß, M., Kogel, K.H., Langen, G., Zorn, H. & Zuccaro, A. (2012) Molecular and phenotypic characterization of Sebacina vermifera strains associated with orchids, and the description of Piriformospora williamsii sp. nov. Fungal Biology 116: 204–213. https://doi.org/10.1016/j.funbio.2011.11.003
- Dearnaley, J., Perotto, S. & Selosse, M.A. (2016) Structure and development of orchid mycorrhizas. In: Martin, F. (Ed.) Molecular Mycorrhizal Symbiosis, 1st ed. John Wiley & Sons, New Jersey, pp. 63–86. https://doi.org/10.1002/9781118951446.ch5
- Deshmukh, S., Hückelhoven, R., Schäfer, P., Imani, J., Sharma, M., Weiss, M., Waller, F. & Kogel, K.H. (2006) The Root Endophytic Fungus Piriformospora indica Requires Host Cell Death for Proliferation during Mutualistic Symbiosis with Barley. Proceedings of the National Academy of Sciences 103 (49): 18450–18457. https://doi.org/10.1073/pnas.0605697103
- Doyle, J.J. & Doyle, J. (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15. https://doi.org/10.2307/2419362
- Franken, P. (2012) The plant strengthening root endophyte Piriformospora indica: potential application and the biology behind. Applied Microbiology and Biotechnology 96: 1455–1464. https://doi.org/10.1007/s00253-012-4506-1
- Fritsche, Y., Lopes, M.E., Selosse, M.A., Stefenon, V.M. & Guerra, M.P. (2021) Serendipita restingae sp. nov. (Sebacinales): an orchid mycorrhizal agaricomycete with wide host range. Mycorrhiza 31: 1–15. https://doi.org/10.1007/s00572-020-01000-7
- Hall, T.A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98.
- Karnovsky, M.J. (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron-microscopy. Journal of Cell Biology 27: 137–138A.
- Kirk, P.M., Stalpers, J.A., Braun, U., Crous, P.W., Hansen, K., Hawksworth, D.L., Hyde, K.D., Lücking, R., Lumbsch, T.H., Rossman, A.Y., Seifert, K.A. & Stadler, M. (2013) A without-prejudice list of generic names of fungi for protection under the International Code of Nomenclature for algae, fungi, and plants. IMA Fungus 4: 381–443. https://doi.org/10.5598/imafungus.2013.04.02.17
- Kottke, I., Beiter, A., Weiss, M., Haug, I., Oberwinkler, F. & Nebel, M. (2003) Heterobasidiomycetes form symbiotic associations with hepatics: Jungermanniales have sebacinoid mycobionts while Aneura pinguis (Metzgeriales) is associated with a Tulasnella species. Mycological Research 107: 957–968. https://doi.org/10.1017/S0953756203008141
- Kottke, I., Suárez, J.P., Herrera, P., Cruz, D., Bauer, R., Haug, I. & Garnica, S. (2010) Atractiellomycetes belonging to the 'rust' lineage (Pucciniomycotina) form mycorrhizae with terrestrial and epiphytic neotropical orchids. Proceedings of the Royal Society B: Biological Sciences 277 (1685): 1289–1298. https://doi.org/10.1098/rspb.2009.1884
- Ma, G.H., Chen, X.G., Selosse, M.A. & Gao, J.Y. (2022) Compatible and Incompatible Mycorrhizal Fungi With Seeds of Dendrobium Species: The Colonization Process and Effects of Coculture on Germination and Seedling Development. Frontiers in Plant Science 13: 823794. https://doi.org/10.3389/fpls.2022.823794
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: 2010 Proceedings of the Gateway Computing Environments Workshop (GCE). New Orleans, Louisiana, USA, pp. 1–8.
- Oberwinkler, F. (1964) Intrahymeniale Heterobasidiomyceten. Fruchtkorperlose Sebacina-Sippen und ihre systematische stellung. Nova Hedwigia 7: 489–499.
- Oberwinkler, F., Riess, K., Bauer, R. & Garnica, S. (2014) Morphology and molecules: the Sebacinales, a case study. Mycological Progress 13: 445–470. https://doi.org/10.1007/s11557-014-0983-1
- Oktalira, F.T., May, T.W., Dearnaley, J.D.W. & Linde, C.C. (2021) Seven new Serendipita species associated with Australian terrestrial orchids. Mycologia 113: 968–987. https://doi.org/10.1080/00275514.2021.1919848
- Qiang, X.Y., Weiss, M., Kogel, K.H. & Schäfer, P. (2012) Piriformospora indica—a mutualistic basidiomycete with an exceptionally large plant host range. Molecular Plant Pathology 13: 508–518. https://doi.org/10.1111/j.1364-3703.2011.00764.x
- Qin, J., Zhang, W., Ge, Z.W. & Zhang, S.B. (2019) Molecular identifications uncover diverse fungal symbionts of pleione (orchidaceae). Fungal Ecology 37: 19–29. https://doi.org/10.1016/j.funeco.2018.10.003
- Rambaut, A. (2018) FigTree v1.4.4. Available from: http://tree.bio.ed.ac.uk/software/figtree/ (accessed: 19 December 2023).
- Rambaut, A. & Drummond, A.J. (2007) Tracer 1.4. Available from: http://beast.bio.ed.ac.uk/Tracer (accessed: 19 December 2023).
- Rasmussen, H.N. (2002) Recent developments in the study of orchid mycorrhiza. Plant and Soil 244: 149–163. https://doi.org/10.1023/A:1020246715436
- Rasmussen, H.N., Dixon, K.W., Jersáková, J. & Těšitelová, T. (2015) Germination and seedling establishment in orchids: a complex of requirements. Annals of Botany 116: 391–402. https://doi.org/10.1093/aob/mcv087
- Ray, P. & Craven, K.D. (2016) Sebacina vermifera: a unique root symbiont with vast agronomic potential. World journal of Microbiology & Biotechnology 32: 16. https://doi.org/10.1007/s11274-015-1970-7
- Riess, K., Oberwinkler, F., Bauer, R. & Garnica, S. (2013) High genetic diversity at the regional scale and possible speciation in Sebacina epigaea and S. incrustans. BMC Evolutionary Biology 13: 102. https://doi.org/10.1186/1471-2148-13-102
- Riess, K., Oberwinkler, F., Bauer, R. & Garnica, S. (2014) Communities of Endophytic Sebacinales Associated with Roots of Herbaceous Plants in Agricultural and Grassland Ecosystems Are Dominated by Serendipita herbamans sp. nov.. PLoS ONE 9: e94676. https://doi.org/10.1371/journal.pone.0094676
- Roberts, P. (1993) Exidiopsis species from Devon, including the new segregate genera Ceratosebacina, Endoperplexa, Microsebacina, and Serendipita. Mycological Research 97: 467–478. https://doi.org/10.1016/S0953-7562(09)80135-4
- Ronquist, F. & Huelsenbeck, J.P. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. https://doi.org/10.1093/bioin forma tics/btg180
- Selosse, M.A., Setaro, S., Glatard, F., Richard, F., Urcelay, C. & Weiß, M. (2007) Sebacinales are common mycorrhizal associates of Ericaceae. New Phytologist 174: 864–878. https://doi.org/10.1111/j.1469-8137.2007.02064.x
- Setaro, S., Weiß, M., Oberwinkler, F. & Kottke, I. (2006) Sebacinales form ectendomycorrhizas with Cavendishia nobilis, a member of the Andean clade of Ericaceae, in the mountain rain forest of southern Ecuador. New Phytologist 169: 355–365. https://doi.org/10.1111/j.1469-8137.2005.01583.x
- Stamatakis, A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. https://doi.org/10.1093/bioin forma tics/btu033
- Suárez, J.P., Weiß, M., Abele, A., Garnica, S., Oberwinkler, F. & Kottke, I. (2006) Diverse tulasnelloid fungi form mycorrhizas with epiphytic orchids in an Andean cloud forest. Mycological Research 110: 1257–1270. https://doi.org/10.1016/j.mycres.2006.08.004
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. & Higgins, D.G. (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. https://doi.org/10.1093/nar/25.24.4876
- Varma, A., Bakshi, M., Lou, B., Hartmanne, A. & Oelmueller, R. (2012) Piriformospora indica: A Novel Plant Growth-Promoting Mycorrhizal Fungus. Agricultural Research 1: 117–131. https://doi.org/10.1007/s40003-012-0019-5
- Verma, S., Varma, A., Rexer, K.H., Hassel, A., Kost, G., Sarbhoy, A., Bisen, P., Bütehorn, B. & Franken, P. (1998) Serendipita indica, gen. et, sp. nov., a new root-colonizing fungus. Mycologia 90: 896. https://doi.org/10.1080/00275514.1998.12026983
- Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
- Wang, X.J., Wu, Y.H., Ming, X.J., Wang, G. & Gao, J.Y. (2021) Isolating ecological-specific fungi and creating fungus-seed bags for epiphytic orchid conservation. Global Ecology and Conservation 28: e01714. https://doi.org/10.1016/j.gecco.2021.e01714
- Warcup, J.H. (1988) Mycorrhizal associations of isolates of Sebacina vermifera. New Phytologist 110: 227–231. https://doi.org/10.1111/j.1469-8137.1988.tb00256.x
- Warcup, J.H. & Talbot, P.H.B. (1967) Perfect states of Rhizoctonias associated with orchids. New Phytologist 66: 631–641. https://doi.org/10.1111/j.1469-8137.1967.tb05434.x
- Weiß, M. & Oberwinkler, F. (2001) Phylogenetic relationships in Auriculariales and related groups—hypotheses derived from nuclear ribosomal DNA sequences. Mycological Research 105: 403–415. https://doi.org/10.1017/s095375620100363x
- Weiß, M., Sýkorová, Z., Garnica, S., Riess, K., Martos, F., Krause, C., Oberwinkler, F., Bauer, R. & Redecker, D. (2011) Sebacinales everywhere: previously overlooked ubiquitous fungal endophytes. PloS ONE 6 (2): e16793. https://doi.org/10.1371/journal.pone.0016793
- Weiß, M., Waller, F., Zuccaro, A. & Selosse, M.A. (2016) Sebacinales–one thousand and one interactions with land plants. New Phytologist 211: 20–40. https://doi.org/10.1111/nph.13977
- Weiss, M., Selosse, M., Rexer, K., Urban, A. & Oberwinkler, F. (2004) Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycological research 108 (9): 1003–1010. https://doi.org/10.1017/s0953756204000772
- Whitehead, M.R., Catullo, R.A., Ruibal, M.P., Dixon, K.W., Peakall, R. & Linde, C.C. (2017) Evaluating multilocus Bayesian species delimitation for discovery of cryptic mycorrhizal diversity. Fungal Ecology 26: 74–84. https://doi.org/10.1016/j.funeco.2016.11.009
- Williams, P.G. (1985) Orchidaceous rhizoctonias in pot cultures of vesiculare-arbuscular mycorrhizal fungi. Canadian Journal of Botany 63: 1329–1333. https://doi.org/10.1139/b85-186
- Xie, L., Long, Y.Y., Zhang, Y., Chen, Y.L. & Zhang, W.L. (2020) Serendipita sacchari sp. nov. from a sugarcane rhizosphere in southern China. Mycotaxon 135: 579–587. https://doi.org/10.5248/135.579
- Xu, Z.X., Zhu, X.M., Yin, H., Li, B., Chen, X.J., Fan, X.L., Li, N.Q., Selosse, M.A., Gao, J.Y. & Han, J.J. (2023) Symbiosis between Dendrobium catenatum protocorms and Serendipita indica involves the plant hypoxia response pathway. Plant Physiology 192: 2554–2568. https://doi.org/10.1093/plphys/kiad198
- Yang, H., Li, N.Q. & Gao, J.Y. (2023) A novel method to produce massive seedlings via symbiotic seed germination in orchids. Frontiers in Plant Science 14: 1114105. https://doi.org/10.3389/fpls.2023.1114105
- Zuccaro, A., Lahrmann, U., Guldener, U., Langen, G., Pfiffi, S., Biedenkopf, D., Wong, P., Samans, B., Grimm, C., Basiewicz, M., Murat, C., Martin, F. & Kogel, K.H. (2011) Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog 7: e1002290. https://doi.org/10.1371/journal.ppat.1002290