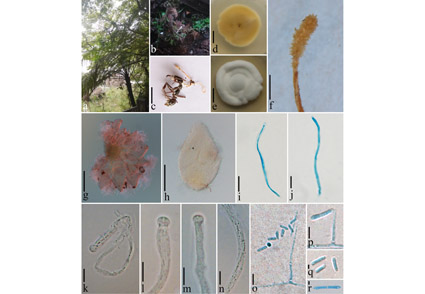
Abstract
A species of entomopathogenic fungi was found on ants in Guizhou Province, China. It is described as Cordyceps poluscapitis (Cordycipitaceae), a novel species with both a sexual and an asexual morph. Cordyceps species are characterized by producing brightly coloured, fleshy stromata and long, filiform ascospores. Cordyceps poluscapitis is unique in that it is parasitic on ants and produces multiple-septate conidia. Multi-gene phylogeny based on combined sequence data of 6-loci genes (ITS, SSU, LSU, TEF, RPB1 and RPB2) confirmed that it is a new species in Cordyceps.
References
Aini, A.N., Mongkolsamrit, S., Wijanarka, W., Thanakitpipattana, D., Luangsa-Ard, J.J. & Budiharjo, A. (2020) Diversity of Akanthomyces on moths (Lepidoptera) in Thailand. MycoKeys 71: 1. https://doi.org/10.3897/mycokeys.71.55126
Andersen, S.B., Gerritsma, S., Yusah, K.M., Mayntz, D., Hywel-Jones, N.L., Billen, J., Boomsma, J.J. & Hughes, D.P. (2009) The life of a dead ant: the expression of an adaptive extended phenotype. The American Naturalist 174 (3): 424–433. https://doi.org/10.1086/603640
Andriolli, F.S., Ishikawa, N.K., Vargas-Isla, R., Cabral, T.S., de Bekker, C. & Baccaro, F.B. (2019) Do zombie ant fungi turn their hosts into light seekers? Behavioral Ecology 30 (3): 609–616. https://doi.org/10.1093/beheco/ary198
Araújo, J.P.M. & Hughes, D.P. (2016) Diversity of entomopathogenic fungi: which groups conquered the insect body? Advances in Genetics 94: 1–39. https://doi.org/10.1016/bs.adgen.2016.01.001
Araújo, J.P.M., Evans, H.C., Kepler, R. & Hughes, D.P. (2018) Zombie-ant fungi across continents: 15 new species and new combinations within Ophiocordyceps. I. Myrmecophilous hirsutelloid species. Studies in Mycology 90: 119–160. https://doi.org/10.1016/j.simyco.2017.12.002
Castlebury, L.A., Rossman, A.Y., Gi-Ho, S., Hyten, A.S. & Spatafora, J.W. (2004) Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research 108 (8): 864–872. https://dx.doi.org/10.1017/S0953756204000607
Chaverri, P., Bischoff, J.F., Evans, H.C. & Hodge, K.T. (2005) Regiocrella, a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 97 (6): 1225–1237. https://doi.org/10.1080/15572536.2006.11832732
Chen, W.H., Han, Y.F., Liang, Z.Q. & Jin, D.C. (2017) Lecanicillium araneogenum sp. nov., a new araneogenous fungus. Phytotaxa 305 (1): 29–34. https://doi.org/10.11646/phytotaxa.305.1.4
Chen, W.H., Liu, M., Huang, Z.X., Yang, G.M., Han, Y.F., Liang, J.D. & Liang, Z.Q. (2018) Beauveria majiangensis, a new entomopathogenic fungus from Guizhou, China. Phytotaxa 333 (2): 243–250. https://doi.org/10.11646/phytotaxa.333.2.8
Chen, W.-H., Han, Y.-F., Liang, J.-D., Tian, W.-Y. & Liang, Z.-Q. (2021) Multi-gene phylogenetic evidence indicates that Pleurodesmospora belongs in Cordycipitaceae (Hypocreales, Hypocreomycetidae) and Pleurodesmospora lepidopterorum sp. nov. on pupa from China. MycoKeys 80: 45–55. https://doi.org/10.3897/mycokeys.80.66794
Evans, H.C. & Samson, R.A. (1982) Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems I. The Cephalotes (Myrmicinae) complex. Transactions of the British Mycological Society 79 (3): 431–453. https://doi.org/10.1016/S0007-1536(82)80037-5
Evans, H.C., Elliot, S.L. & Hughes, D.P. (2011) Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. PloS one 6 (3): e17024. https://doi.org/10.1371/journal.pone.0017024
Evans, H.C., Araújo, J.P.M., Halfeld, V.R. & Hughes, D.P. (2018) Epitypification and re-description of the zombie-ant fungus, Ophiocordyceps unilateralis (Ophiocordycipitaceae). Fungal Systematics and Evolution 1 (1): 13–22. https://doi.org/10.3114/fuse.2018.01.02
Flakus, A., Etayo, J., Miadlikowska, J., Lutzoni, F., Kukwa, M., Matura, N. & Rodriguez-Flakus, P. (2019) Biodiversity assessment of ascomycetes inhabiting Lobariella lichens in Andean cloud forests led to one new family, three new genera and 13 new species of lichenicolous fungi. Plant and Fungal Systematics 64 (2): 283–344. https://doi.org/10.2478/pfs-2019-0022
Hall, T., Biosciences, I. & Carlsbad, C. (2011) BioEdit: an important software for molecular biology. GERF Bull Biosci 2 (1): 60–61.
Hughes, D.P., Andersen, S.B., Hywel-Jones, N.L., Himaman, W., Billen, J. & Boomsma, J. (2011) Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecology 11 (1): 1–10. https://doi.org/10.1186/1472-6785-11-13
Humber, R.A., Rocha, L.F.N., Inglis, P.W., Kipnis, A. & Luz, C. (2013) Morphology and molecular taxonomy of Evlachovaea-like fungi, and the status of this unusual conidial genus. Fungal Biology 117 (1): 1–12. https://doi.org/10.1016/j.funbio.2012.10.001
Hyde, K.D., de Silva, N.I., Jeewon, R., Bhat, D.J., Phookamsak, R., Doilom, M., Boonmee, S., Jayawardena, R.S., Maharachchikumbura, S.S.N., Senanayake, I.C., Manawasinghe, I.S., Liu, N.G., Abeywickrama, P.D., Chaiwan, N., Karunarathna, A., Pem, D., Lin, C.G., Sysouphanthong, P., Luo, Z.L., Wei, D.P., Wanasinghe, D.N., Norphanphoun, C., Liu, J.K., Tennakoon, D.S., Samarakoon, M.C., Jayasiri, S.C., Jiang, H.B., Zeng, X.Y., Li, J.F., Wijesinghe, S.N., Devadatha, B., Goonasekara, I.D., Brahmanage, R.S., Yang, E.F., Aluthmuhandiram, J.V.S., Dayarathne, M.C., Marasinghe, D.S., Li, W.J., Dissanayake, L.S., Dong, W., Huanraluek, N., Lumyong, S., Karunarathna, S.C., Jones, E.B.G., Al-Sadi, A.M., Xu, J.C., Harishchandra, D., Sarma, V.V. & Bulgakov, T.S. (2020) AJOM new records and collections of fungi: 1–100. Asian Journal of Mycology 3 (1): 22–294. https://doi.org/10.5943/ajom/3/1/3
Johnson, D., Sung, G.-H., Hywel-Jones, N.L., Luangsa-ard, J.J., Bischoff, J.F., Kepler, R.M. & Spatafora, J.W. (2009) Systematics and evolution of the genus Torrubiella (Hypocreales, Ascomycota). Mycological Research 113 (3): 279–289. https://doi.org/10.1016/j.mycres.2008.09.008
Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30 (4): 772–780. https://doi.org/10.1093/molbev/mst010
Kepler, R.M., Sung, G.H., Ban, S., Nakagiri, A., Chen, M.J., Huang, B., Li, Z. & Spatafora, J.W. (2012) New teleomorph combinations in the entomopathogenic genus Metacordyceps. Mycologia 104 (1): 182–197. https://doi.org/10.3852/11-070
Kepler, R.M., Luangsa-ard, J.J., Hywel-Jones, N.L., Quandt, C.A., Sung, G.H., Rehner, S.A., Aime, M.C., Henkel, T.W., Sanjuan, T., Zare, R., Chen, M., Li, Z., Rossman, A.Y., Spatafora, J.W. & Shrestha, B. (2017) A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 8 (2): 335–353. https://doi.org/10.5598/imafungus.2017.08.02.08
Kobayasi, Y. (1981) Revision of the genus Cordyceps and its allies 2. Bulletin of the National Science Museum. Series B, Botany 7 (4): 123–129.
Kobayasi, Y. & Shimizu, D. (1978) Cordyceps Species from Japan. Bulletin of the National Science Museum. Series B, Botany 4 (2): 43–63.
Kouvelis, V.N., Sialakouma, A. & Typas, M.A. (2008) Mitochondrial gene sequences alone or combined with ITS region sequences provide firm molecular criteria for the classification of Lecanicillium species. Mycological Research 112 (7): 829–844. https://doi.org/10.1016/j.mycres.2008.01.016
Li, G.J., Hyde, K.D., Zhao, R.L., Hongsanan, S., Abdel-Aziz, F.A., Abdel-Wahab, M.A., Alvarado, P., Alves-Silva, G., Ammirati, J.F., Ariyawansa, H.A., Baghela, A., Bahkali, A.H., Beug, M., Bhat, D.J., Bojantchev, D., Boonpratuang, T., Bulgakov, T.S., Camporesi, E., Boro, M.C., Ceska, O., Chakraborty, D., Chen, J.J., Chethana, K.W.T., Chomnunti, P., Consiglio, G., Cui, B.K., Dai, D.Q., Dai, Y.C., Daranagama, D.A., Das, K., Dayarathne, M.C., De Crop, E., De Oliveira, R.J.V., Fragoso de Souza, C.A., de Souza, J.I., Liu, J.K., Dentinger, B.T.M., Dissanayake, A.J., Doilom, M., Drechsler-Santos, E.R., Ghobad-Nejhad, M., Gilmore, S.P., Goes-Neto, A., Gorczak, M., Haitjema, C.H., Hapuarachchi, K.K., Hashimoto, A., He, M.Q., Henske, J.K., Hirayama, K., Iribarren, M.J., Jayasiri, S.C., Jayawardena, R.S., Jeon, S.J., Jernimo, G.H., Jesus, A.L., Jones, E.B.G., Kang, J.C., Karunarathna, S.C., Kirk, P.M., Konta, S., Kuhnert, E., Langer, E., Lee, H.S., Lee, H.B., Li, W.J., Li, X.H., Liimatainen, K., Lima, D.X., Lin, C.G., Liu, X.., Liu, Z.Y., Wen, T.C., Luangsa-ard, J.J., Luecking, R., Lumbsch, H.T., Lumyong, S., Leano, E.M., Marano, A.V., Matsumura, M., McKenzie, E.H.C., Mongkolsamrit, S., Mortimer, P.E., Thuong, N.T.T., Niskanen, T., Norphanphoun, C., O’Malley, M.A., Parnmen, S., Pawlowska, J., Perera, R.H., Phookamsak, R., Phukhamsakda, C., Pires-Zottarelli, C.L.A., Raspe, O., Reck, M.A., Rocha, S.C.O., de Santiago, A.L.C.M.A., Senanayake, I.C., Setti, L., Shang, Q.J., Singh, S.K., Sir, E.B., Solomon, K.V., Song, J., Srikitikulchai, P., Stadler, M., Suetrong, S., Takahashi, H., Takahashi, T., Tanaka, K., Tang, L.P., Yang, J., Thambugala, K.M., Thanakitpipattana, D., Theodorou, M.K., Thongbai, B., Wen, H.A., Thummarukcharoen, T., Tian, Q., Tibpromma, S., Verbeken, A., Vizzini, A., Vlasak, J., Voigt, K., Wanasinghe, D.N., Wang, Y., Weerakoon, G., Wijayawardene, N.N., Wongkanoun, S., Wrzosek, M., Xiao, Y.P., Xu, J.C., Yan, J.Y., Yang, S.D., Hu, Y., Zhang, J.F., Zhao, J., Zhou, L.W., Persoh, D., Phillips, A.J.L. & Maharachchikumbura, S.S.N. (2016) Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 78 (1): 1–237. https://doi.org/10.1007/s13225-016-0366-9
Li, Y.P., Chen, W.H., Han, Y.F., Liang, J.D. & Liang, Z.Q. (2020) Cordyceps yinjiangensis, a new ant-pathogenic fungus. Phytotaxa 453 (3): 284–292. https://doi.org/10.11646/phytotaxa.453.3.10
Liang, Z.Q., Liu, A.Y., Huang, J.Z. & Jiao, Y.C. (2002) Some Cordyceps species and their allies from Suoluo nature preserve in Guizhou. Mycosystema 21 (1): 9–14.
Luangsa-ard, J.J., Hywel-Jones, N.L., Manoch, L. & Samson, R.A. (2005) On the relationships of Paecilomyces sect. Isarioidea species. Mycological Research 109 (5): 581–589. https://doi.org/10.1017/s0953756205002741
Massee, G. (1891) A new Cordyceps. Annals of Botany 5 (1): 510–511. https://doi.org/10.1093/oxfordjournals.aob.a090653
Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Gateway Computing Environments Workshop (GCE). pp. 1–8. https://doi.org/10.1109/GCE.2010.5676129
Mongkolsamrit, S., Noisripoom, W., Thanakitpipattana, D., Wutikhun, T., Spatafora, J.W. & Luangsa-ard, J.J. (2018) Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 110 (1): 230–257. https://orcid.org/0000-0001-6801-2145
Mongkolsamrit, S., Noisripoom, W., Arnamnart, N., Lamlertthon, S., Himaman, W., Jangsantear, P., Samson, R.A. & Luangsa-ard, J.J. (2019) Resurrection of Paraisaria in the Ophiocordycipitaceae with three new species from Thailand. Mycological Progress 18 (9): 1213–1230. https://doi.org/10.1007/s11557-019-01518-x
Mongkolsamrit, S., Noisripoom, W., Tasanathai, K., Khonsanit, A., Thanakitpipattana, D., Himaman, W., Kobmoo, N. & Luangsa-ard, J.J. (2020) Molecular phylogeny and morphology reveal cryptic species in Blackwellomyces and Cordyceps (Cordycipitaceae) from Thailand. Mycological Progress 19 (9): 957–983. https://doi.org/10.1007/s11557-020-01615-2
Mongkolsamrit, S., Noisripoom, W., Tasanathai, K., Kobmoo, N., Thanakitpipattana, D., Khonsanit, A., Petcharad, B., Sakolrak, B. & Himaman, W. (2022) Comprehensive treatise of Hevansia and three new genera Jenniferia, Parahevansia and Polystromomyces on spiders in Cordycipitaceae from Thailand. MycoKeys 91: 113–149. https://doi.org/10.3897/ mycokeys.91.83091
Moureau, J. (1961) Nouveaux Cordyceps du Congo. Lejeunia Memoirs 15: 1–38.
Moureau, J. & Lacquemant, S. (1949) Cordyceps du Congo belge. Georges van Campenhout.
Qu, J., Yu, L.Q., Zhang, J., Han, Y. & Zou, X. (2018) A new entomopathogenic fungus, Ophiocordyceps ponerus sp. nov., from China. Phytotaxa 343 (2): 116–126. https://doi.org/10.11646/phytotaxa.343.2.2
Rambaut, A. (2012) Figtree 1.4.0. Available from: http://tree.bio.ed.ac.uk/software/figtree/ (accessed 13 April 2022)
Rehner, S.A. & Buckley, E. (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97 (1): 84–98. https://dx.doi.org/10.3852/mycologia.97.1.84
Rehner, S.A., Minnis, A.M., Sung, G.-H., Luangsa-ard, J.J., Devotto, L. & Humber, R.A. (2011) Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 103 (5): 1055–1073. https://doi.org/10.3852/10-302
Saccardo, P.A. (1878) Fungi italici autographice delineati a Prof. PA Saccardo. Patavii 1878. Fascicoli V-VIII sistentes tab. 161–320. Michelia 1: 326–350.
Sanjuan, T., Tabima, J., Restrepo, S., Læssøe, T., Spatafora, J.W. & Franco-Molano, A.E. (2014) Entomopathogens of Amazonian stick insects and locusts are members of the Beauveria species complex (Cordyceps sensu stricto). Mycologia 106 (2): 260–275. https://doi.org/10.3852/106.2.260
Simmons, D.R., Lund, J., Levitsky, T. & Groden, E. (2015) Ophiocordyceps myrmicarum, a new species infecting invasive Myrmica rubra in Maine. Journal of Invertebrate Pathology 125: 23–30. https://doi.org/10.1016/j.jip.2014.12.010
Spatafora, J.W., Quandt, C.A., Kepler, R.M., Sung, G.-H., Shrestha, B., Hywel-Jones, N.L. & Luangsa-ard, J.J. (2015) New 1F1N species combinations in Ophiocordycipitaceae (Hypocreales). IMA Fungus 6 (2): 357–362. https://doi.org/10.5598/imafungus.2015.06.02.07
Sun, Y.B. (2017) FasParser: a package for manipulating sequence data. Zoological Research 38 (2): 110. https://doi.org/10.24272/j.issn.2095-8137.2017.017
Sung, G.H., Hywel-Jones, N.L., Sung, J.M., Luangsa-ard, J.J., Shrestha, B. & Spatafora, J.W. (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology 57: 5–59. https://doi.org/10.3114/sim.2007.57.01
Tasanathai, K., Thanakitpipattana, D., Noisripoom, W., Khonsanit, A., Kumsao, J. & Luangsa-ard, J.J. (2016) Two new Cordyceps species from a community forest in Thailand. Mycological Progress 15 (3): 1–8. https://doi.org/10.1007/s11557-016-1170-3
Thanakitpipattana, D., Tasanathai, K., Mongkolsamrit, S., Khonsanit, A., Lamlertthon, S. & Luangsa-ard, J.J. (2020) Fungal pathogens occurring on Orthopterida in Thailand. Persoonia 44 (1): 140–160.
Thanakitpipattana, D., Mongkolsamrit, S., Khonsanit, A., Himaman, W., Luangsa-Ard, J.J. & Pornputtapong, N. (2022) Is Hyperdermium Congeneric with Ascopolyporus? Phylogenetic Relationships of Ascopolyporus spp. (Cordycipitaceae, Hypocreales) and a New Genus Neohyperdermium on Scale Insects in Thailand. Journal of Fungi 8 (5): 516. https://doi.org/10.3390/jof8050516
Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172 (8): 4238–4246. https://dx.doi.org/10.1128/jb.172.8.4238-4246
Vinit, K., Doilom, M., Wanasinghe, D.N., Bhat, D.J., Brahmanage, R.S., Jeewon, R., Xiao, Y.P. & Hyde, K.D. (2018) Phylogenetic placement of Akanthomyces muscarius, a new endophyte record from Nypa fruticans in Thailand. Current Research in Environmental & Applied Mycology 8 (3): 404–417. https://doi.org/10.5943/cream/8/3/10
Wang, Y.B., Wang, Y., Fan, Q., Duan, D.E., Zhang, G.D., Dai, R.Q., Dai, Y.D., Zeng, W.B., Chen, Z.H., Li, D.D., Tang, D.X., Xu, Z.H, Sun, T., Thi-Tra, N., Ngoc-Lan, T., Van-Minh, D., Zhang, C.M., Huang, L.D., Liu, Y.J., Zhang, X.M., Yang, D.R., Sanjuan, T., Liu, X.Z., Yang, Z.L. & Yu, H. (2020) Multigene phylogeny of the family Cordycipitaceae (Hypocreales): new taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Diversity 103 (1): 1–46. https://doi.org/10.1007/s13225-020-00457-3
Wen, T.C., Xiao, Y.P., Li, W.J., Kang, J.C. & Hyde, K.D. (2014) Systematic analyses of Ophiocordyceps ramosissimum sp. nov., a new species from a larvae of Hepialidae in China. Phytotaxa 161 (3): 227–234. https://doi.org/10.11646/phytotaxa.161.3.6
Wen, T.C., Xiao, Y.P., Zha, L.S., Hyde, K.D. & Kang, J.C. (2016) Multigene phylogeny and morphology reveal a new species, Ophiocordyceps tettigonia, from Guizhou Province, China. Phytotaxa 280 (2): 141–151. http://dx.doi.org/10.11646/phytotaxa.280.2.4
White, T.J., Bruns, S., Lee, S. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, T.J. (Eds.) PCR protocols: a guide to methods and applications. Academic Press Inc., New York, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Xiao, Y.P., Wang, Y.B., Hyde, K.D. Eleni, G., Sun, J.Z., Yang, Y., Meng, J., Yu, H. & Wen, T.C. (2023) Polycephalomycetaceae, a new family of clavicipitoid fungi segregates from Ophiocordycipitaceae. Fungal Diversity. https://doi.org/10.1007/s13225-023-00517-4
Xiao, Y.P., Wen, T.C., Hongsanan, S., Sun, J.Z. & Hyde, K.D. (2017) Introducing Ophiocordyceps thanathonensis, a new species of entomogenous fungi on ants, and a reference specimen for O. pseudolloydii. Phytotaxa 328 (2): 115–126. https://doi.org/10.11646/phytotaxa.328.2.2
Zare, R. & Gams, W. (2016) More white verticillium-like anamorphs with erect conidiophores. Mycological Progress 15 (10–11): 993–1030. https://doi.org/10.1007/s11557-016-1214-8
Zhang, Z.F., Zhou, S.Y., Eurwilaichitr, L., Ingsriswang, S., Raza, M., Chen, Q., Zhao, P., Liu, F & Cai, L. (2021) Culturable mycobiota from Karst caves in China II, with descriptions of 33 new species. Fungal Diversity 106 (1): 29–136. https://doi.org/10.1007/s13225-020-00453-7