Abstract
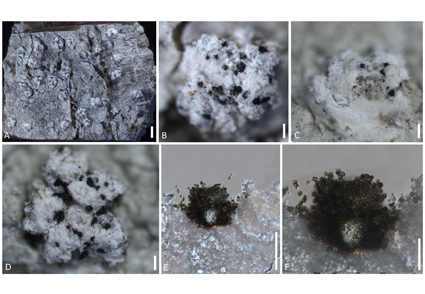
Sclerococcum is a species rich lichenicolous genus in Ascomycota. However, many species of Sclerococcum have been described based only on morphology. This study provides sequences for the large-subunit ribosomal RNA (LSU), internal transcribed spacer (ITS) and mitochondrial small-subunit ribosomal RNA (mtSSU) for the first time for Sclerococcum simplex, and the first geographical record of the genus from China. Phylogenetic analyses confirmed that S. simplex clustered within Sclerococcum sensu stricto while the genus was recovered as paraphyletic in Dactylosporaceae. Our asexual specimen of Sclerococcum simplex was collected on a corticolous Pertusaria thallus. The taxonomic affinity of Sclerococcum simplex is investigated based on phylogenetic and morphological evidence.
References
Diederich, P. & van den Boom, P.P.G. (2013) Two new lichenicolous species of Hainesia (asexual Ascomycetes) growing on Cladonia. Bulletin de la Societé des naturalistes luxembourgeois 114: 59–64.
Diederich, P. & van den Boom, P.P.G. (2017) Sclerococcum phaeophysciae and S. toensbergii, two new lichenicolous asexual Ascomycetes, with a revised key to the species of Sclerococcum. Bulletin de la Société des naturalistes luxembourgeois 119: 71–78.
Diederich, P. (1990) New or interesting lichenicolous fungi 1. Species from Luxembourg. Mycotaxon 37: 297–330.
Diederich, P. (2010) Sclerococcum cladoniae, a new lichenicolous hyphomycete on Cladonia from Luxembourg. Bulletin de la Societé des naturalistes luxembourgeois 111: 57–59.
Diederich, P. (2015) Two new lichenicolous species of Sclerococcum (asexual Ascomycetes) growing on Graphidaceae. Bulletin de la Société des naturalistes luxembourgeois 117: 35–42.
Diederich, P., Ertz, D., Lawrey, J.D., Sikaroodi, M. & Untereiner, W.A. (2013) Molecular data place the hyphomycetous lichenicolous genus Sclerococcum close to Dactylospora (Eurotiomycetes) and S. parmeliae in Cladophialophora (Chaetothyriales). Fungal Diversity 58: 61–72. https://doi.org/10.1007/s13225-012-0179-4
Diederich, P. & van den Boom, P. (2017) Sclerococcum phaeophysciae and S. toensbergii, two new lichenicolous asexual Ascomycetes, with a revised key to the species of Sclerococcum. Bulletin de la Société des naturalistes luxembourgeois 119: 71–78.
Diederich, P., Lawrey, J.D. & Ertz, D. (2018) The 2018 classification and checklist of lichenicolous fungi, with 2000 non-lichenized, obligately lichenicolous taxa. Bryologist 121: 340–425. https://doi.org/10.1639/0007-2745-121.3.340
Dissanayake, A.J., Bhunjun, C.S., Maharachchikumbura, S.S.N. & Liu, J.K. (2020) Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere 11 (1): 2652–2676. https://doi 10.5943/mycosphere/11/1/18
Dong, W., Hyde, K.D., Doilom, M., Yu, X.D., Bhat, D.J., Jeewon, R., Boonmee, S., Wang, G.N., Nalumpang, S. & Zhang, H. (2020) Pseudobactrodesmium (Dactylosporaceae, Eurotiomycetes, Fungi) a novel lignicolous genus. Frontiers in Microbiology 11: 1–13. https://doi.org/10.3389/fmicb.2020.00456
Ekanayaka, A.H., Jones, E.G., Hyde, K.D. & Zhao, Q. (2019) A stable phylogeny for Dactylosporaceae. Cryptogamie Mycologie 40 (3): 23–44. https://doi.org/10.5252/cryptogamie-mycologie2019v40a3
Etayo, J. & Diederich, P. (1995) Lichenicolous fungi from the western Pyrenees, France and Spain. I. Flechten Follmann. Contributions to lichenology in honour of Gerhard Follmann 205–221.
Fries, E.M. (1819) Novitiae florae suecicae 5. Lund.
Fries, E.M. (1825) Systema orbis vegetabilis I. Lund.
GenBank (2021) Available from: https://www.ncbi.nlm.nih.gov/nuccore/ (accessed 6 April 2021)
Glez-Peña, D., Gómez-Blanco, D., Reboiro-Jato, M., Fdez-Riverola, F. & Posada, D. (2010) ALTER: program-oriented conversion of DNA and protein alignments. Nucleic Acids Research 38: 14–18. https://doi.org/10.1093/nar/gkq321
Hawksworth, D.L. (1979) The lichenicolous Hyphomycetes. Bulletin of the British Museum (Natural History). Botany Series 6: 183–300.
Huelsenbeck, J.P. & Ronquist, F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Index Fungorum (2021) Available from: http://www.indexfungorum.org/names/Names.asp (accessed 6 April 2021)
Jayasiri, S.C., Hyde, K.D., Ariyawansa, H.A., Bhat, J., Buyck, B., Cai, L., Dai, Y.C., Abd-Elsalam, K.A., Ertz, D., Hidayat, I., Jeewon, R., Jones, E.B.G., Bahkali, A.H., Karunarathna, S.C., Liu, J.K., Luangsa-ard, J.J., Lumbsch, H.T. Maharachchikumbura, S.S.N., McKenzie, E.H.C., Moncalvo, J.M., Ghobad-Nejhad, M., Nilsson, H., Pang, K.A., Pereira, O.L., Phillips, A.J.L., Raspé, O., Rollins, A.W., Romero, A.I., Etayo, J., Selçuk, F., Stephenson, S.L., Suetrong, S., Taylor, J.E., Tsui, C.K.M., Vizzini, A., Abdel-Wahab, M.A., Wen, T.C., Boonmee, S., Dai, D.Q., Daranagama, D.A., Dissanayake, A.J., Ekanayaka, A.H., Fryar, S.C., Hongsanan, S., Jayawardena, R.S., Li, W.J., Perera, R.H., Phookamsak, R., de Silva, N.I., Thambugala, K.M., Tian, Q., Wijayawardene, N.N., Zhao, R.L., Zhao, Q., Kang, J.C. & Promputtha, I. (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Diversity 74 (1): 3–18. https://doi.org/10.1007/s13225-015-0351-8
Joshi, Y. (2021) Two new species of lichenicolous fungus Sclerococcum (Dactylosporaceae, Sclerococcales) from India. Acta Botanica Hungarica 63: 67–75. https://doi.org/10.1556/034.63.2021.1-2.5
Joshi, Y., Falswal, A., Tripathi, M., Upadhyay, S., Bisht, A., Chandra, K., Bajpai, R. & Upreti, D.K. (2016) One hundred and five species of lichenicolous biota from India: An updated checklist for the country. Mycosphere 7: 268–294. https://doi.org/10.5943/mycosphere/7/3/3
Katoh, K. & Standley, K. (2013) MAFFT Multiple Sequence Alignment Software Version 7: improvements in performance and usability. Molecular Biology. Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010
Lawrey, J.D., Binder, M., Diederich, P., Molina, M.C., Sikaroodi, M. & Ertz, D. (2007) Phylogenetic diversity of lichen-associated homobasidiomycetes. Molecular Phylogenetics and Evolution 44 (2): 778–789. https://doi.org/10.1016/j.ympev.2006.12.023
Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010) Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In: SC10 Workshop on Gateway Computing Environments (GCE10). https://doi.org/10.1109/GCE.2010.5676129
Nylander, J.A.A. (2004) MrAIC. pl. Program distributed by the author. Sweden: Evolutionary Biology Centre, Uppsala University.
Olariaga, I., Teres, J., Martín, J., Prieto, M. & Baral, H.O. (2019) Pseudosclerococcum golindoi gen. et sp. nov., a new taxon with apothecial ascomata and a Chalara-like anamorph within the Sclerococcales (Eurotiomycetes). Mycological Progress 18: 895–905. https://doi.org/10.1007/s11557-019-01500-7
Pang, K.L., Guo, S.Y., Alias, S.A., Hafellner, J. & Jones, E.B.G. (2014) A new species of marine Dactylospora and its phylogenetic affinities within the Eurotiomycetes, Ascomycota. Botanica Marina 57: 315–321. https://doi.org/10.1515/bot-2014-0025
Pino-Bodas, R., Zhurbenko, M.P. & Stenroos, S. (2017) Phylogenetic placement within Lecanoromycetes of lichenicolous fungi associated with Cladonia and some other genera. Persoonia 39: 91–117.
Rambaut, A. (2012) FigTree version 1.4.0. Available at http://tree.bio.ed.ac.uk/software/figtree/ (accessed 1 April 2021)
Réblová, M., Untereiner, W.A., Št?pánek, V. & Gams, W. (2017) Disentangling Phialophora section Catenulatae: disposition of taxa with pigmented conidiophores and recognition of a new subclass, Sclerococcomycetidae (Eurotiomycetes). Mycological Progress 16: 27–46. https://doi.org/10.1007/s11557-016-1248-y
Senanayake, I.C., Rathnayake, A.R., Marasinghe, D.S., Calabon, M.S., Gentekaki, E., Lee, H.B., Hurdeal, V.G., Pem, D., Dissanayake, L.S., Wijesinghe, S., Bundhun, D., Nguyen, T.T., Goonasekara, I.D., Abeywickrama, P.D., Bhunjun, C.S., Jayawardena, R.S., Wanasinghe, D.N., Jeewon, R., Bhat, D.J. & Xiang, M.M. (2020) Morphological approaches in studying fungi: collection, examination, isolation, sporulation and preservation. Mycosphere 11 (1): 2678–2754. https://doi 10.5943/mycosphere/11/1/20
Stamatakis, A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
van den Boom, P.P.G., Breuss, O., Spier, L. & Brand, A. (1996) Beitrag zur Flechtenflora Kärntens. Ergebnisse der Feldtagung der bryologischen und lichenologischen Arbeitsgruppe der KNNV in Weissbriach. Linzer biologische Beiträge 28: 619–654.
Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
Wijayawardene, N.N., Hyde, K.D., Al-Ani, L.K.T., Tedersoo, L., Haelewaters, D., Rajeshkumar, K.C., Zhao, R.L., Aptroot, A., Leontyev, D.V., Saxena, R.K., Tokarev, Y.S., Dai, D.Q, Letcher, P.M, Stephenson, S.L., Ertz, D., Lumbsch, H.T., Kukwa, M., Issi, I.V., Madrid, H., Phillips, A.J.L, Selbmann, L, Pfliegler, W.P, Horváth, E, Bensch, K., Kirk, P., Kola?íková, Z., Raja, H.A., Radek, R, Papp, V., Dima, B., Ma, J., Malosso, E., Takamatsu, S., Rambold, G., Gannibal, P.B., Triebel, D., Gautam, A.K., Avasthi, S., Suetrong, S., Timdal, E., Fryar, S.C., Delgado, G., Réblová, M., Doilom, M., Dolatabadi, S., Paw?owska, J., Humber, R.A., Kodsueb, R., Sánchez-Castrov, I., Goto, B.T., Silva, D.K.A., de Souza, F.A., Oehl, F., da Silva, G.A., Silva, I.R., B?aszkowski, J., Jobim, K., Maia, L.C., Barbosa, F.R., Fiuza, P.O., Divakar, P.K., Shenoy, B.D., Castañeda-Ruiz, R.F., Somrithipol, S., Karunarathna, S.C., Tibpromma, S., Mortimer,.PE., Wanasinghe, D.N., Phookamsak, R., Xu, J., Wang, Y., Fenghua, T., Alvarado, P., Li, D.W., Kušan, I., Mato?ec, N., Maharachchikumbura, S.S.N., Papizadeh, M., Heredia, G., Wartchow, F., Bakhshi, M., Boehm, E., Youssef, N., Hustad, V.P., Lawrey, J.D., Santiago, A.L.C.M., Bezerra, J.D.P., Souza-Motta, C.M., Firmino, A.L., Tian, Q., Houbraken, J., Hongsanan, S., Tanaka, K., Dissanayake, A.J., Monteiro, J.S., Grossart, H.P., Suija, A., Weerakoon, G., Etayo, J., Tsurykau, A., Kuhnert, E., Vázquez, V., Mungai, P., Damm, U., Li, Q.R., Zhang, H., Boonmee, S., Lu, Y.Z., Becerra AG., Kendrick, B., Brearley FQ., Motiej?naitë, J., Sharma, B., Khare, R., Gaikwad, S., Wijesundara, D.S.A., Tang, L.Z., He, M.Q., Flakus, A., Rodriguez-Flakus, P., Zhurbenko, M.P., McKenzie, E.H.C., Stadler, M., Bhat, D.J., Kui-Liu, J., Raza, M., Jeewon, R., Nassonova, E.S., Prieto, M., Jayalal, R.G.U., Erdoðdu, M., Yurkov, A., Schnittler, M., Shchepin, O.N., Novozhilov, Y.K., Silva-Filho, AGS., Gentekaki, E., Liu, P., Cavender, J.C., Kang, Y., Mohammad, S., Zhang, L.F., Xu, R.F., Li, Y.M., Dayarathne, M.C., Ekanayaka, A.H., Wen, T.C., Deng, C.Y., Lateef, A.A., Pereira, O.L., Navathe, S., Hawksworth, D.L., Fan, X.L., Dissanayake, L.S., Kuhnert, E., Grossart, H.P. & Thines, M. (2020) Outline of fungi and fungus-like taxa. Mycosphere 11: 1060–1456. https://doi.org/10.5943/mycosphere/11/1/8
Yu, X.D., Dong, W., Bhat, D.J., Boonmee, S., Zhang, D.I. & Zhang, H. (2018) Cylindroconidiis aquaticus gen. et sp. nov., a new lineage of aquatic hyphomycetes in Sclerococcaceae (Eurotiomycetes). Phytotaxa 372: 79–87. https://doi.org/10.11646/phytotaxa.372.1.6
Zhurbenko, M.P. (2004) Lichenicolous and some interesting lichenized fungi from the Northern Ural, Komi Republic of Russia. Herzogia 17: 77–86.
Zoller, S., Scheidegger, C. & Sperisen, C. (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31: 511–516. https://doi.org/10.1006/lich.1999.0220