Abstract
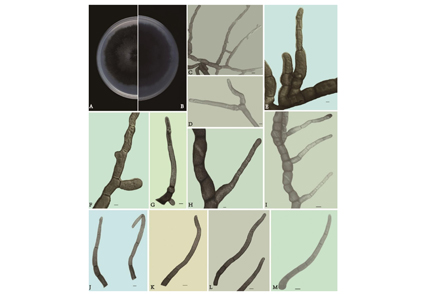
The genus Phaeomycocentrospora (Dothidotthiaceae) is a fungal pathogen causing plant leaf spots. A new species, Phaeomycocentrospora xinjangensis sp. nov. is introduced from Xinjiang Uygur Autonomous Region, Northwest China. Multi-gene phylogenetic analyses based on a concatenated ITS, LSU, and ACT sequence dataset were used to confirm the phylogenetic position of the new species. Phaeomycocentrospora xinjangensis can easily be distinguished from the remaining species based on multi-gene phylogenetic analyses coupled with morphological characters. Morphologically, P. xinjangensis differs from other species in the genus by the presence of dark hyphae, dark brown conidiophores, few branched conidiogenous cells. Phylogenetically, our three strains were clustered together and formed a separate subclade with high support values. Moreover, we provided a description, illustrations, and phylogenetic tree for the new species.
References
Carbone, I., Anderson, J.B. & Kohn, L.M. (1999) Patterns of descent in clonal lineages and their multilocus fingerprints are resolved with combined gene genealogies. Evolution 53: 11–21. https://10.1111/j.15585646.1999.tb05329.x
Crous, P.W., Braun, U., Hunter, G.C., Wingfield, M.J., Verkley, G.J.M., Shin, H.D., Nakashima, C. & Groenewald, J.Z. (2013) Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. https://doi.org/10.3114/sim0005
Drummond, A. & Rambaut, A. (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC evolutionary biology 7: 214–221. https://doi.org/10.1186/1471-2148-7-214
Hongsanan, S., Hyde, K.D., Phookamsak, R., Wanasinghe, D.N., McKenzie, E.H.C., Sarma, V.V. & Xie, N. (2020) Refined families of dothideomycetes: dothideomycetidae and pleosporomycetidae. Mycosphere 11: 1553–2107. https://doi.org/10.5943/mycosphere/11/1/13
Katoh, K. & Standley, D.M. (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010
Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K., von Haeseler, A. & Jermiin, L.S. (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589. https://doi.org/10.1038/nmeth.4285
Li, X., Zhang, Z.Y., Chen, W.H., Liang, J.D., Huang, J.Z., Han, Y.F. & Liang, Z.Q. (2022) A new species of Arthrographis (Eremomycetaceae, Dothideomycetes), from the soil in Guizhou, China. Phytotaxa 538: 175–181. https://doi.org/10.11646/phytotaxa.538.3.1
Minh, Q., Nguyen, M. & von Haeseler, A.A. (2013) Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution 30: 1188–1195. https://doi.org/10.1093/molbev/mst024
Marin-Felix, Y., Groenewald, J.Z., Cai, L., Chen, Q. & Crous, P.W. (2017) Genera of phytopathogenic fungi: gophy 1. Studies in Mycology 86: 99–216. https://doi.org/10.1016/j.simyco.2017.04.002
Nguyen, L.T., Schmidt, H.A., von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. https://doi.org/10.1093/molbev/msu300
Posada, D. & Crandall, K.A. (1998) Modeltest: testing the model of DNA substitution. Bioinformatics,14: 817–818. https://doi.org/10.1093/bioinformatics/14.9.817
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
Rehner, S.A. & Samuels, G.J. (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. https://doi.org/10.1016/S0953-7562(09)80409-7
Senwanna, C., Wanasinghe, D.N., Bulgakov, T.S., Wang, Y., Bhat, D.J., Tang, A.M.C. & Phookamsak, R. (2019) Towards a natural classification of Dothidotthia and Thyrostroma in Dothidotthiaceae (Pleosporineae, Pleosporales). Mycosphere 10: 701–738. https://doi.org/10.5943/mycosphere/10/1/15
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. https://doi.org/10.1093/molbev/mst197
Tullio, V., Banche, G., Allizond,V., Roana, J., Mandras, N., Scalas, D., Panzone, M., Cervetti, O., Valle, S., Carlone, N. & Cuffini, A.M. (2010) Non-dermatophyte moulds as skin and nail foot mycosis agents: Phoma herbarum, Chaetomium globosum and Microascus cinereus. Fungal Biology 114: 345–349. https://doi.org/10.1016/j.funbio.2010.02.003
Vaidya, G., Lohman, D.J. & Meier, R. (2011) Sequence Matrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27: 171–180. https://doi.org/10.1111/j.1096-0031.2010.00329.x
Vilgalys, R. & Hester, M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC213247/pdf/jbacter00122-0118]
White, T.J., Bruns, T., Lee, S.J.W.T. & Taylor, J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: a guide to methods and amplications 18: 315–322. [https://msafungi.org/wp-content/uploads/2019/03/February-2013-Inoculum]
Zhang, Z.Y., Han, Y.F., Chen, W.H. & Liang, Z.Q. (2019) Gongronella sichuanensis (Cunninghamellaceae, Mucorales), a new species isolated from soil in China. Phytotaxa 416: 167–174. https://doi.org/10.11646/Phytotaxa.416.2.4
Zhang, Z.Y., Shao, Q.Y., Li, X., Chen, W.H., Liang, J.D., Han, Y.F., Huang, J.Z. & Liang, Z.Q. (2021) Culturable fungi from urban soils in China I: description of 10 new taxa. Microbiology Spectrum 9: e00867-21. https://doi.org/10.1128/Spectrum.00867-21