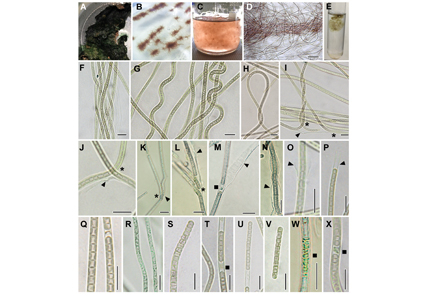
Abstract
Cyanobacterial diversity has been extensively studied; however, scant knowledge exists concerning genetic data and higher accuracy for tropical cyanobacterial diversity. In particular, the existence of unnamed monophyletic genera within the Oculatellaceae requires further investigation. Here, two novel strains (NUACC13 and NUACC14) were isolated from a cyanobacterial mat covering border soil of a small freshwater pond in Phitsanulok Province, Thailand. A polyphasic approach combining genetic (16S rDNA and rpoC1 gene phylogenies, evolutionary distance and ITS secondary structures) and morphological information was applied to evaluate the taxonomic status of these novel strains. All phylogenetic analyses of 16S rDNA and rpoC1 gene sequences placed our strains within the Oculatellaceae, strongly supporting a novel monophyletic clade that did not belong to other known Oculatellaceae genera. The 16S rDNA sequence similarity (<96.2%) values among the closest described genera also supported the novelty of our strains. The hypothetical secondary structures (D1–D1′, Box-B, V2 and V3 helices) of the ITS region confirmed the uniqueness of our strains, indicating differences from the related genera. Morphological features of the novel strains differed from the phylogenetically closest taxa by size and color of filament and vegetative cell and the presence of a frayed sheath. Considering all the results, we herein described our strains as the novel genus Siamcapillus gen. nov. in the Oculatellaceae with the type species S. rubidus sp. nov.
References
Basheva, D., Moten, D., Stoyanov, P., Belkinova, D., Mladenov, R. & Teneva, I. (2018) Content of phycoerythrin, phycocyanin, alophycocyanin and phycoerythrocyanin in some cyanobacterial strains: Applications. Engineering in Life Sciences 18: 861–866. https://doi.org/10.1002/elsc.201800035
Bennett, A. & Bogorad, L. (1973) Complementary chromatic adaptation in a filamentous blue-green alga. Journal of Cell Biology 58: 419–435. https://doi.org/10.1083/jcb.58.2.419
Berthold, D.E., Lefler, F.W. & Laughinghouse, H.D. (2021) Untangling filamentous marine cyanobacterial diversity from the coast of South Florida with the description of Vermifilaceae fam. nov. and three new genera: Leptochromothrix gen. nov., Ophiophycus gen. nov., and Vermifilum gen. nov. Molecular Phylogenetics and Evolution 160: 107010. https://doi.org/10.1016/j.ympev.2020.107010
Berrendero Gómez, E., Johansen, J.R., Kaštovský, J., Bohunická, M. and ?apková, K. (2016) Macrochaete gen. nov. (Nostocales, Cyanobacteria), a taxon morphologically and molecularly distinct from Calothrix. Journal of Phycology 52: 638–655. https://doi.org/10.1111/jpy.12425
Brito, Â., Ramos, V., Mota, R., Lima, S., Santos, A., Vieira, J., Vieira, C.P., Kaštovský, J., Vasconcelos, V.M. & Tamagnini, P. (2017) Description of new genera and species of marine cyanobacteria from the Portuguese Atlantic coast, Molecular Phylogenetics and Evolution 111: 18–34. https://doi.org/10.1016/j.ympev.2017.03.006.
Brown, I.I., Bryant, D.A., Casamatta, D., Thomas-Keprta, K.L., Sarkisova, S.A., Shen, G., Graham, J.E., Boyd, E.S., Peters, J.W., Garrison, D.H. & McKay, D.S. (2010) Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits. Applied Environmental Microbiology 76: 6664–6672. https://doi.org/10.1128/AEM.00662-10
Casamatta, D., Johansen, J.R., Vis, M.L. & Broadwater, S.T. (2005) Molecular and morphological characterization of ten polar and near polar strains within the Oscillatoriales (Cyanobacteria). Journal of Phycology 41: 421–438. https://doi.org/10.1111/j.1529-8817.2005.04062.x
Chakraborty, S., Maruthanayagam, V., Achari, A., Pramanik, A., Jaisankar, P. & Mukherjee, J. (2021) Aerofilum fasciculatum gen. nov., sp. nov. (Oculatellaceae) and Euryhalinema pallustris sp. nov. (Prochlorotrichaceae) isolated from an Indian mangrove forest. Phytotaxa 522: 165–186. https://doi.org/10.11646/phytotaxa.522.3.1
Chan, P.P., & Lowe, T.M. (2019) tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. Methods in molecular biology (Clifton, N.J.) 1962: 1–14. https://doi.org/10.1007/978-1-4939-9173-0_1
Charpy, L., Casareto, B.E., Langlade, M.J. & Suzuki, Y. (2012) Cyanobacteria in coral reef ecosystems: A review. Journal of Marine Biology 2012: 1–9. https://doi.org/10.1155/2012/259571
Chatchawan, T., Komárek, J., Strunecky, O., Smarda, J. & Peerapornpisal, Y. (2012) Oxynema, a new genus separated from the genus Phormidium (Cyanophyta). Cryptogamie Algologie 33: 41–59. https://doi.org/10.7872/crya.v33.iss1.2011.041
Demay, J., Bernard, C. Reinhardt, A. & Marie, B. (2019) Natural products from cyanobaceria: Focus on beneficial activities. Marine Drugs 17: 320. https://doi.org/10.3390/md17060320
Drancourt, M., Bollet, C., Carlioz, A., Martelin, R., Gayral, J.P. & Raoult, D. (2000) 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial Isolates. Journal of Clinical Microbiology 38: 3623–3630. https://doi.org/10.1128/JCM.38.10.3623-3630.2000
Dvo?ák, P., Poulí?ková, A., Hašler, P., Belli, M., Casamatta, D.A. & Papini, A. (2015) Species concepts and speciation factors in cyanobacteria, with connection to the problems of diversity and classification. Biodiversity and Conservation 24: 739–757. https://doi.org/10.1007/s10531-015-0888-6
Dvo?ák, P., Hašler, P., Casamatta, D.A. & Poulí?ková, A. (2021) Underestimated cyanobacteria diversity: trends and perspectives of research in tropical environments. Fottea 21: 110–127. https://doi.org/10.0.21.131/fot.2021.009
Edgar, R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. https://doi.org/10.1093/nar/gkh340
Genuário, D.B., Vas, M.G.M.V., Hentschke, G.S., Sant’Anna, C.L. & Fiore, M.F. (2015) Halotia gen. nov., a phylogenetically and physiologically coherent cyanobacterial genus isolated from marine coastal environments. International Journal of Systematic and Evolutionary Microbiology 65: 663–675. https://doi.org/10.1099/ijs.0.070078-0
González-Resendiz, L., Johansen, J.R., Alba-Lois, L., Segal-Kischinevzky, C., Escobar-Sámche, V., Jiménez-García, L.F., Huaer, T. & León-Tejera, H. (2018) Nunduva, a new marine genus of Rivulariaceae (Nostocales, Cyanobacteria) from marine rocky shores. Fottea 18: 86–105. https://doi.org/10.5507/fot.2017.018
González-Resendiz, L., Johansen, J.R., León-Tejera, H., Sánchez, L., Segal-Kischinevzky, C., Escobar-Sánchez, V. & Morales, M. (2019) A bridge too far in naming species: a total evidence approach does not support recognition of four species in Desertifilum (Cyanobacteria). Journal of Phycology 55: 898–911. https://doi.org/10.1111/jpy.12867
Guindon, S., Dufayard, J-F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010) New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Systematic Biology 59: 307–321. https://doi.org/10.1093/sysbio/syq010
Hašler, P., Dvo?ák, P., Johansen, J.R., Kitner, M., Ond?ej, V. & Poulí?ková, A. (2012) Morphological and molecular study of epipelic filamentous genera Phormidium, Microcoleus and Geitlerinema (Oscillatoriales, Cyanophyta/Cyanobacteria). Fottea 12: 341–356. https://doi.org/10.5507/fot.2012.024
Hašler, P., Dvo?ák, P., Poulí?ková, P. & Casamatta, D.A. (2014) A novel genus Ammassolinea gen. nov. (Cyanobacteria) isolated from sub-tropical epipelic habitats. Fottea 14: 241–248. https://doi.org/10.5507/fot.2014.018
Hauerová, R., Hauer, T., Kaštovský, J., Komárek, J., Lepšová-Skácelová, O. & Mareš, O. (2021) Tenebriella gen. nov. – The dark twin of Oscillatoria, Molecular Phylogenetics and Evolution 165: 107293. https://doi.org/10.1016/j.ympev.2021.107293.
Iteman, I., Rippka, R., de Marsac, N.T. & Herdman, M. (2000) Comparison of conserved structural and regulatory domains within divergent 16S rRNA–23S rRNA spacer sequences of cyanobacteria. Microbiology 146: 1275–1286. https://doi.org/10.1099/00221287-146-6-1275
Jahodá?ová, E., Dvo?ák, P., Hašler, P., Holušová, K. & Poulí?ková, A. (2018) Elainella gen. nov.: a new tropical cyanobacterium characterized using a complex genomic approach. European Journal of Phycology 53: 39–51 https://doi.org/10.1080/09670262.2017.1362591
Johansen, J.R. & Casamatta, D.A. (2005) Recognizing cyanobacterial diversity through adoption of a new species paradigm. Algological Studies 117: 71–93. https://doi.org/10.1127/1864-1318/2005/0117-0071
Johansen, J.R, Kovácik, L., Casamatta, D.A., Fu?iková, K. & Kaštovský, J. (2011) Utility of 16S-23S ITS sequence and secondary structure for recognition of intrageneric and intergeneric limits within cyanobacterial taxa: Leptolyngbya corticola sp. nov. (Pseudanabaenaceae, Cyanobacteria). Nova Hedwigia 92: 283–302. https://doi.org/10.1127/0029-5035/2011/0092-0283
Johansen, J.R., González-Resendiz, L., Escobar-Sánchez, V., Segal-Kischinevzky, C., Martínez-Yerena, J., Hernández-Sánchez, J., Hernández-Pérez, G. & León-Tejera, H. (2021) When will taxonomic saturation be achieved? A case study in Nunduva and Kyrtuthrix (Rivulariaceae, Cyanobacteria). Journal of Phycology 57 (6): 1699–1720. https://doi.org/10.1111/jpy.13201
Jungblut, A., Lovejoy, C. & Vincent, W. (2010) Global distribution of cyanobacterial ecotypes in the cold biosphere. The ISME Journal 4: 191–202. https://doi.org/10.1038/ismej.2009.113
Komárek, J. (2006) Cyanobacterial Taxonomy: Current problems and prospects for the integration of traditional and molecular approaches. Algae 21: 349–375. https://doi.org/10.4490/algae.2006.21.4.349
Komárek, J. & Anagnostidis, K. (2005) Cyanoprokaryota.2.Oscillatoriales. In: Büdel, B., Krienitz, L., Gärtner, G. & Schagerl, M. (Eds.) Süsswasserflora von Mitteleuropa 19/2. Elsevier/Spektrum, Heidelberg. pp. 759.
Kaštovský, J., Berrendero Gomez, E., Hladil, J. & Johansen, J.R. (2014) Cyanocohniella calida gen. et sp. nov. (Cyanobacteria: Aphanizomenonaceae) a new cyanobacterium from the thermal springs from Karlovy Vary, Czech Republic. Phytotaxa 181: 279–292. https://doi.org/10.11646/phytotaxa.181.5.3
Komárek, J., Kaštovsky´, J., Mareš, J. & Johansen, J.R. (2014) Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 86: 295–335. [http://www.preslia.cz/P144Komarek.pdf]
Komárek, J. (2016) A polyphasic approach for the taxonomy of cyanobacteria: principles and applications. European Journal of Phycology 51: 346–353. https://doi.org/10.1080/09670262.2016.1163738
Komárek, J. (2018) Several problems of the polyphasic approach in the modern cyanobacterial system. Hydrobiologia 811: 7–17. https://doi.org/10.1007/s10750-017-3379-9
Kozlíková-Zapom?lová, E., Chatchawan, T., Kaštovský, J., & Komárek, J. (2016) Phylogenetic and taxonomic position of the genus Wollea with the description of Wollea salina sp. nov. (Cyanobacteria, Nostocales). Fottea 16: 43–55. https://doi.org/10.5507/fot.2015.026
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular Biology and Evolution 35: 1547–1549. https://doi.org/10.1093/molbev/msy096
Lyons, T.W., Reinhard, C.T. & Planavsky, N.J. (2014) The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506: 307–315. https://doi.org/10.1038/nature13068
Mai, T., Johansen, J.R., Pietrasiak, N., Bohunicka, M. & Martin, M.P. (2018) Revision of the Synechococcales (Cyanobacteria) through recognition of four families including Oculatellaceae fam. nov. and Trichocoleaceae fam. nov. and six new genera containing 14 species. Phytotaxa 365: 1–59. https://doi.org/10.11646/phytotaxa.365.1.1
Malone, C.F.d.S., Genuário, D.B., Vaz, M.G.M.V., Fiore, M.F. & Sant’Anna, C.L. (2021) Monilinema gen. nov., a homocytous genus (Cyanobacteria, Leptolyngbyaceae) from saline–alkaline lakes of Pantanal wetlands, Brazil. Journal of Phycology 57: 473–483. https://doi.org/10.1111/jpy.13106
Mareš, J., Johansen, J.R., Hauer, T., Zima Jr., J., Ventura, S., Cuzman, O., Tiribilli, B & Kaštovský, J. (2019) Taxonomic resolution of the genus Cyanothece (Chroococcales, Cyanobacteria), with a treatment on Gloeothece and three new genera, Crocosphaera, Rippkaea, and Zehria. Journal of Phycology 55: 578?610. https://doi.org/10.1111/jpy.12853
Mathews Lab, University of Rochester Medical Center (2018) RNA structure, version 6.0.1 Available from: https://rna.urmc.rochester.edu/RNAstructure.html (accessed 16 April 2018)
Mühlsteinová, R., Johansen, J.R., Pietrasiak, N., Martin, M.P., Osoirio-Santos, K. & Warren, S.D. (2014) Polyphasic characterization of Trichocoleus desertorum sp. nov. Pseudanabaenales, Cyanobacteria) from desert soils and phylogenetic placement of the genus Trichocoleus. Phytotaxa 163: 241–261. https://doi.org/10.11646/phytotaxa.163.5.1
Neilan, B.A., Jacobs, D., Dot, T.D., Blackall, L.L., Hawkins, P.R., Cox, P.T. & Goodman, A.E. (1997) rRNA Sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. International Journal of Systematic Bacteriology 47: 693–697. https://doi.org/10.1099/00207713-47-3-693
Nowruzi, B., & Shalygin, S. (2021) Multiple phylogenies reveal a true taxonomic position of Dulcicalothrix alborzica sp. nov. (Nostocales, Cyanobacteria). Fottea 21: 235–246. https://doi.org/10.5507/fot.2021.008
Nübel, U., Garcia-Pichel, F. & Muyzer, G. (1997) PCR primers to amplify 16S rRNA genes from cyanobacteria. Applied and Environmental Microbiology 63: 3327–3332. https://doi.org/10.1128/aem.63.8.3327-3332.1997
Nylander, J.A.A. (2008) mrmodeltest v2. Program distributed by author. Evolutionary Biology Centre, Uppsala University.
Osorio-Santos, K., Pietrasiak, N., Bohunická, M., Miscoe, L.H., Kovacik, L., Martin, M.P. & Johansen, J.R. (2014) Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria) European Journal of Phycology 49: 450–470. https://doi.org/10.1080/09670262.2014.976843
Pagel, F., Guedes, A.C., Amaro, H.M., Kijjoa, A. & Vasconcelos, V. (2019) Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnology Advances 37: 422–443. https://doi.org/10.1016/j.biotechadv.2019.02.010.
Partensky, F., Six, C., Ratin, M., Garczarek, L., Vaulot, D., Probert, I., Calteau, A., Gourvil, P., Marie, D., Grébert, T., Bouchier, C., Le Panse, S., Gachenot, M., Rodríguez, F. & Garrido, J.L. (2018) A novel species of the marine cyanobacterium Acaryochloris with a unique pigment content and lifestyle. Scientific Report 8: 9142. https://doi.org/10.1038/s41598-018-27542-7
Pietrasiak, N., Muhlsteinova, R., Siegesmund, M.A. & Johansen, J.R. (2014) Phylogenetic placement of Symplocastrum (Phormidiaceae) with a new combination S. californicum and two new species: S. flechtnerae and S. torsivum. Phycologia 53: 529–541. https://doi.org/10.2216/14-029.1
Pietrasiak, N., Osorio-Santos, K., Shalygin, S., Martin, M.P. & Johansen, J.R. (2019) When is a lineage a species? A case study in Myxacorys gen. nov. (Synechococcales: Cyanobacteria) with the description of two new species from the Americas. Journal of Phycology 55: 976–996. https://doi.org/10.1111/jpy.12897
Pietrasiak, N., Reeve, S., Osorio-Santos, K., Lipson, D.A. & Johansen, J.R. (2021) Trichotorquatus gen. nov. - a new genus of soil cyanobacteria discovered from American drylands. Journal of Phycology 57: 886–902. https://doi.org/10.1111/jpy.13147
Philippot, L., Andersson, S.G., Battin, T.J., Prosser, J.I., Schimel, J.P., Whitman, W.B. & Hallin, S. (2010) The ecological coherence of high bacterial taxonomic ranks. Nature Reviews Microbiology 8: 523–529. https://doi.org/10.1038/nrmicro2367
Sciuto, K. & Moro, I. (2016) Detection of the new cosmopolitan genus Thermoleptolyngbya (Cyanobacteria, Leptolyngbyaceae) using the 16S rRNA gene and 16S–23S ITS region. Molecular Phylogenetics and Evolution 105: 15–35. https://doi.org/10.1016/j.ympev.2016.08.010
Sciuto, K., Moschin, E. & Moro, I. (2017) Cryptic Cyanobacterial Diversity in the Giant Cave (Trieste, Italy): The New Genus Timaviella (Leptolyngbyaceae). Cryptogamie Algologie 38: 285–323. https://doi.org/10.7872/crya/v38.iss4.2017.285
Sendall, B.C. & McGregor, G.B. (2018) Cryptic diversity within the Scytonema complex: Characterization of the paralytic shellfish toxin producer Heterosyctonema crispum, and the establishment of the family Heteroscytonemataceae (Cyanobacteria/Nostocales). Harmful Algae 80: 158–170. https://doi.org/10.1016/j.hal.2018.11.002.
Seo, P-S. & Yokota, A. (2003) The phylogenetic relationships of cyanobacteria inferred from 16S rRNA, gyrB, rpoC1 and rpoD1 gene sequences. The journal of General and Applied Microbiology 49: 191–203. https://doi.org/10.2323/jgam.49.191
Shen, L.-Q., Zhang, Z.-C., Shang, J.-L., Li, Z.-K., Chen, M., Li, R. & Qiu, B.-S. (2022) Kovacikia minuta sp. nov. (Leptolyngbyaceae, Cyanobacteria), a new freshwater chlorophyll f-producing cyanobacterium. Journal of Phycology 58 (3): 424–435. https://doi.org/10.1111/jpy.13248
Strunecky, O., Bohunická, M., Johansen, J.R., ?apková, K., Raabová, L., Dvo?ák, P., & Komárek, J. (2017) A revision of the genus Geitlerinema and a description of the genus Anagnostidinema gen. nov. (Oscillatoriophycidae, Cyanobacteria). Fottea 17: 114–126. https://doi.org/10.5507/fot.2016.025
Strunecky, O., Raabova, L., Bernardova, A., Ivanova, A.P., Semanova, A., Crossley, J. & Kaftan, D. (2020) Diversity of cyanobacteria at the Alaska North Slope with description of two new genera: Gibliniella and Shackletoniella, FEMS Microbiology Ecology 96: fiz189. https://doi.org/10.1093/femsec/fiz189
Strunecky, O., Kopejtka, K., Goecke, F., Tomasch, J., Lukavský, J., Neori, A., Kahl, S., Pieper, D.H., Pilarski, P., Kaftan, D. & Koblížek, M. (2019) High diversity of thermophilic cyanobacteria in Rupite hot spring identified by microscopy, cultivation, single-cell PCR and amplicon sequencing. Extremophiles 23: 35–48. https://doi.org/10.1007/s00792-018-1058-z.
Ronquist, F., Teslenko, M. van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
Tang, W., Cerdán-García, E., Berthelot, H., Polyviou, D., Wang, S., Baylay, A., Whitby, H., Planquette, H., Mowlem, M., Robidart, J., & Cassar, N. (2020) New insights into the distributions of nitrogen fixation and diazotrophs revealed by high-resolution sensing and sampling methods. The ISME journal 14: 2514–2526. https://doi.org/10.1038/s41396-020-0703-6
Tawong, W. (2017) Diversity of the Potential 2-Methylisoborneol-Producing Genotypes in Thai Strains of Planktothricoides (Cyanobacteria). Brazilian Archives of Biology and Technology 60: e17160567. https://doi.org/10.1590/1678-4324-2017160567
Tawong, W., Pongcharoen, P., Pongpadung, P. & Ponza, S. (2019a) Neowollea manoromense sp. nov. (Nostocales, Cyanobacteria), a novel geosmin producer isolated from Thailand. Phytotaxa 424: 1–17.
Tawong, W., Pongcharoen, P., Nishimura, T. & Adachi, M. (2019b) Molecular characterizations of Thai Raphidiopsis raciborskii (Nostocales, Cyanobacteria) based on 16S rDNA, rbcLX, and cylindrospermopsin synthetase genes. Plankton and Benthos Research 14: 211–223. https://doi.org/10.3800/pbr.14.211
Wang, Y., Cai, F., Jia, N. & Li, R. (2019) Description of a novel coccoid cyanobacterial genus and species Sinocapsa zengkensis gen. nov. sp. nov. (Sinocapsaceae, incertae sedis), with taxonomic notes on genera in Chroococcidiopsidales. Phytotaxa 409: 146–160. https://doi.org/10.11646/phytotaxa.409.3.3
Yarza, P., Yilmaz, P., Pruesse, E., Glöckner, F.O., Ludwig, W., Schleifer, K.-H., Whitman, W.B., Euzéby, J., Rudolf, A. & Rosselló-Móra, R. (2014) Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nature Reviews Microbiology 12: 635–645. https://doi.org/10.1038/nrmicro3330
Zammit, G., Billi, D. & Albertano, P. (2012) The subaerophytic cyanobacterium Oculatella subterranea (Oscillatoriales, Cyanophyceae) gen. et sp. nov.: a cytomorphological and molecular description. European Journal of Phycology 47: 341–354. https://doi.org/10.1080/09670262.2012.717106
Zammit, G. (2018) Systematics and biogeography of sciophilous cyanobacteria; an ecological and molecular description of Albertania skiophila (Leptolyngbyaceae) gen. & sp. nov. Phycologia 57: 481–491. https://doi.org/10.2216/17-125.1