Abstract
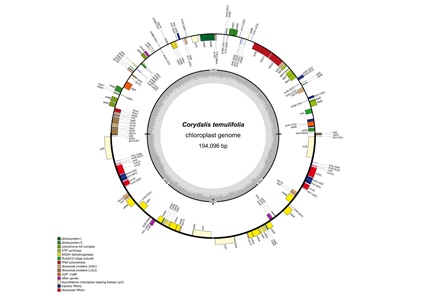
Corydalis temulifolia has been considered to be one of the primitive species of Corydalis; however, some recent works based on DNA markers revealed instead that Corydalis adunca rather C. temulifolia is in the basal clade within the Corydalis lineage. Both views are in conflict over concerns of species representing the primitive member of Corydalis. In this study, the chloroplast genome of C. temulifolia has been sequenced and compared with previously published chloroplast genome of C. adunca. With the sequences obtained, genomic phylogenetic analyses have been carried out. Although sharing the quadripartite structure of C. adunca, the complete chloroplast genome of C. temulifolia has particular features with respect to its genome size, gene order, gene content, and nucleotide substitution rates. For instance, we found the complete chloroplast genome of C. temulifolia has SSC region of 341 bp, which is markedly different in size than that of C. adunca (9,531 bp). C. temulifolia was determined as a species with large-scale rearrangements, consisting of the relocation of segment (~5 kb, trnV-UAC-rbcL) in the LSC region, the inversion of segment (~14 kb, ndhB-trnR-ACG) in the IR region and a large size (>10 kb) of IR expansion in IRs/SSC junction. Significantly, of the four large-scale rearrangements the relocation of the segment (trnV-UAC-rbcL) was absent from C. adunca but other two rearrangements were shared by C. adunca. In the latter, the segment (trnV-UAC-rbcL) resided in the conserved location and has not occurred rearrangement, suggesting that C. adunca was most likely represent a relatively early divergence species in Corydalis that compared to C. temulifolia. Further, we noticed that there was no change to the gene order of rps16 and rrn16 in C. temulifolia relative to C. adunca, and unlike C. adunca that seven out of ndh genes reside as pseudogenes, 11 ndh genes are all present in C. temulifolia. In addition, C. temulifolia has not only more dispersed repeats but slightly more tandem repeats and simple sequence repeats than C. adunca. The selection pressure estimation of protein-coding genes (psaI and rps7) in C. temulifolia were under positive selection, and it is different from four genes (psaI, rpl23, rpl36 and rps7) in C. adunca. Phylogenetic analysis based on the plastid genome revealed C. adunca to be the basal clade within the Corydalis lineage in the present sampling, suggesting that C. temulifolia is most likely not representative of the primitive members of Corydalis. This might indicate that the importance of morphological characters such as stigma may be in part misinterpreted, when being used to make inference about the evolutionary status of the Corydalis species. The results emphasise the need to consider both morphological and molecular evidence when determining not only the systematic position of species but also character evolution.
References
Alzahrani, D.A., Yaradua, S.S., Yaradua, S.S., Albokhari, E.J., Albokhari, E.J. & Abba, A. (2020) Complete chloroplast genome sequence of Barleria prionitis, comparative chloroplast genomics and phylogenetic relationships among Acanthoideae. BMC Genomics 21 (1): 1–19. https://doi.org/10.1186/s12864-020-06798-2
Benson, G. (1999) Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Research 27 (2): 573–580. https://doi.org/10.1093/nar/27.2.573
Bunting. (1966) In: Baileya 13: 172.
Chen, S.F., Zhou, Y.Q., Chen, Y.R. & Gu, J. (2018) Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34 (17): i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Cho, W.B., Lee, D.H., Choi, I.S. & Lee, J.H. (2018) The complete chloroplast genome of hemi-parasitic Pedicularis hallaisanensis (Orobanchaceae). Mitochondrial DNA Part B: Resources 3 (1): 235–236. https://doi.org/10.1080/23802359.2018.1437820
Cock, P.J.A., Antao, T., Chang, J.T., Chapman, B.A., Cox, C.J., Dalke, A., Friedberg, I., Hamelryck, T., Kauff, F., Wilczynski, B. & De Hoon, M.J.L. (2009) Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25 (11): 1422–1423. https://doi.org/10.1093/bioinformatics/btp163
Cosner, M.E., Raubeson, L.A. & Jansen, R.K. (2004) Chloroplast DNA rearrangements in Campanulaceae: Phylogenetic utility of highly rearranged genomes. BMC Evolutionary Biology 4: 1–17. https://doi.org/10.1186/1471-2148-4-27
Darling, A.C.E., Mau, B., Blattner, F.R. & Perna, N.T. (2004) Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Research 14 (7): 1394–1403. https://doi.org/10.1101/gr.2289704
Darriba, D. & Posada, D. (2012) jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9 (8): 772. https://doi.org/10.1038/nmeth.2109
Delannoy, E., Fujii, S., Colas Des Francs-Small, C., Brundrett, M. & Small, I. (2011) Rampant Gene loss in the underground orchid Rhizanthella Gardneri highlights evolutionary constraints on plastid genomes. Molecular Biology and Evolution 28 (7): 2077–2086. https://doi.org/10.1093/molbev/msr028
Dierckxsens, N., Mardulyn, P. & Smits, G. (2016) NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Research 45 (4): 1–9. https://doi.org/10.1093/nar/gkw955
Doyle, J.J. & Doyle, J.L. (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15.
Fay, J.C. & Wu, C.I. (2003) Sequence Divergence, Functional Constraint, and Selection in Protein Evolution. Annual Review of Genomics and Human Genetics 4: 213–235. https://doi.org/10.1146/annurev.genom.4.020303.162528
Fedde, F. (1924) Neue Arten von Corydalis aus China, ?. Repertorium Specierum Novarum Regni Vegetabilis 20: 50–63. https://doi.org/10.1002/fedr.19240200109
Fedde, F. (1925) Neue Arten von Corydalis aus China, IX. Repertorium Specierum Novarum Regni Vegetabilis 21: 46–52. https://doi.org/10.1002/fedr.19250210107
Fedde, F. (1925) Neue Arten von Corydalis aus China, X. Repertorium Specierum Novarum Regni Vegetabilis 22: 26. https://doi.org/10.1002/fedr.19250220105
Firetti, F., Zuntini, A.R., Gaiarsa, J.W., Oliveira, R.S., Lohmann, L.G. & Van Sluys, M.A. (2017) Complete chloroplast genome sequences contribute to plant species delimitation: A case study of the Anemopaegma species complex. American Journal of Botany 104 (10): 1493–1509. https://doi.org/10.3732/ajb.1700302
Franchet, A.R. (1894) Plantes Nouvelles de la Chine occidentale. Journal de Botanique 8: 273–304.
Franchet, F.M. (1886) Nouvelles Archives du Muséum d’Histoire Naturelle. Paris, sér. 2, 8: 198.
Franch. (1886) In: Nouvelles archives du Muséum d’histoire naturelle Ser. 2, 8: 198.
Frazer, K.A., Pachter, L., Poliakov, A., Rubin, E.M. & Dubchak, I. (2004) VISTA: computational tools for comparative genomics. Nucleic Acids Research 32: W273–W279. https://doi.org/10.1093/nar/gkh458
Greiner, S., Lehwark, P. & Bock, R. (2019) OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Research 47 (W1): W59–W64. https://doi.org/10.1093/nar/gkz238
Guisinger, M.M., Chumley, T.W., Kuehl, J.V., Boore, J.L. & Jansen, R.K. (2010) Implications of the plastid genome sequence of Typha (Typhaceae, Poales) for understanding genome evolution in Poaceae. Journal of Molecular Evolution 70 (2): 149–166. https://doi.org/10.1007/s00239-009-9317-3
Guisinger, M.M., Kuehl, J.V., Boore, J.L. & Jansen, R.K. (2011) Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: Rearrangements, repeats, and codon usage. Molecular Biology and Evolution 28 (1): 583–600. https://doi.org/10.1093/molbev/msq229
Hong, C.P., Park, J., Lee, Y., Lee, M., Park, S.G., Uhm, Y., Lee, J. & Kim, C.K. (2017) accD nuclear transfer of Platycodon grandiflorum and the plastid of early Campanulaceae. BMC Genomics 18 (1): 607. https://doi.org/10.1186/s12864-017-4014-x
Hook, f. & Thomson. (1864) On the Genus Euptela, Sieb. & Zucc. Journal of the Proceedings of the Linnean Society, Botany 7: 243. [London]
Jin, J.J., Yu, W.B., Yang, J.B., Song, Y., Claude, W. & Yi, T.S. (2020) GetOrganelle?: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biology 21: 241. https://doi.org/10.1186/s13059-020-02154-5
Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30 (4): 772–780. https://doi.org/10.1093/molbev/mst010
Kim, K.J. & Lee, H.L. (2004) Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Research 11 (4): 247–261. https://doi.org/10.1093/dnares/11.4.247
Kurtz, S., Choudhuri, J.V., Ohlebusch, E., Schleiermacher, C., Stoye, J. & Giegerich, R. (2001) REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Research 29 (22): 4633–4642. https://doi.org/10.1093/nar/29.22.4633
Lamarck, J.B. & Candolle, A.P. (1805) Flore Française, ed. 3, 4 (2). Chez H. Agasse, Paris, 545 pp.
Lee, H.L., Jansen, R.K., Chumley, T.W. & Kim, K.J. (2007) Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. Molecular Biology and Evolution 24 (5): 1161–1180. https://doi.org/10.1093/molbev/msm036
Lee, C., Choi, I.S., Cardoso, D., de Lima, H.C., de Queiroz, L.P., Wojciechowski, M.F., Jansen, R.K. & Ruhlman, T.A. (2021) The chicken or the egg? Plastome evolution and an independent loss of the inverted repeat in papilionoid legumes. Plant Journal 107 (3): 861–875. https://doi.org/10.1111/tpj.15351
Li, H.T., Yi, T.S., Gao, L.M., Ma, P.F., Zhang, T., Yang, J.B., Gitzendanner, M.A., Fritsch, P.W., Cai, J., Luo, Y., Wang, H., van der Bank, M., Zhang, S.D., Wang, Q.F., Wang, J., Zhang, Z.R., Fu, C.N., Yang, J., Hollingsworth, P.M., Chase, M.W., Soltis, D.E., Soltis, P.S & Li, D.Z. (2019) Origin of angiosperms and the puzzle of the Jurassic gap. Nature Plants 5 (5): 461–470. https://doi.org/10.1038/s41477-019-0421-0
Li, H.T., Luo, Y., Gan, L., Ma, P.F., Gao, L.M., Yang, J.B., Cai, J., Gitzendanner, M.A., Fritsch, P.W., Zhang, T., Jin, J.J., Zeng, C.X., Wang, H., Yu, W.B., Zhang, R., van der Bank, M., Olmstead, R.G., Hollingsworth, P.M., Chase, M.W. & Soltis, D.E. (2021) Plastid phylogenomic insights into relationships of all flowering plant families. BMC Biology 19 (1): 232. [13 pp.] https://doi.org/10.1186/s12915-021-01166-2
Li, X., Yang, J.B., Wang, H., Song, Y., Corlett, R.T., Yao, X., Li, D.Z. & Yu, W.B. (2021) Plastid NDH Pseudogenization and Gene Loss in a Recently Derived Lineage from the Largest Hemiparasitic Plant Genus Pedicularis (Orobanchaceae). Plant and Cell Physiology 62 (6): 971–984. https://doi.org/10.1093/pcp/pcab074
Lidén, M., Fukuhara, T. & Axberg, T. (1995) Phylogeny of Corydalis, ITS and morphology. Systematics and Evolution of the Ranunculiflorae, 188: 183–188. https://doi.org/10.1007/978-3-7091-6612-3_17
Lidén, M., Fukuhara, T., Rylander, J. & Oxelman, B. (1997) Phylogeny and classification of Fumariaceae, with emphasis on Dicentra s. l., based on the plastid gene rps16 intron. Plant Systematics and Evolution 206 (1–4): 411–420. https://doi.org/10.1007/bf00987960
Lidén, M. (1989) Corydalis (Papaveraceae: Fumarioideae) in Nepal. Bull. Bulletin of the British Museum (Natural History) Botany 18 (6): 524.
Lidén, M. (1986) In: Opera Bot 88: 28.
Linnaeus, C. (1753) POLYANDRIA. MONOGYNIA, Classis XIII. In: Species Plantarum, vol. 1. 508 pp.
Linnaeus, C. (1753) DIDYNAMIA ANGIOSPERMIA. In: Species Plantarum, vol. 2. 636 pp. https://doi.org/10.5962/bhl.title.669
Linnaeus, C. von. (1753) Species Plantarum 2: 723.
Liu, Y., Huo, N., Dong, L., Wang, Y., Zhang, S., Young, H.A., Feng, X. & Gu, Y.Q. (2013) Complete Chloroplast Genome Sequences of Mongolia Medicine Artemisia frigida and Phylogenetic Relationships with Other Plants. PLoS ONE 8 (2): e57533. https://doi.org/10.1371/journal.pone.0057533
Liu, L.X., Du, Y.X., Shen, C., Li, R., Lee, J. & Li, P. (2020) The complete chloroplast genome of papaver setigerum and comparative analyses in papaveraceae. Genetics and Molecular Biology 43 (3): 1–10. https://doi.org/10.1590/1678-4685-GMB-2019-0272
Luo, Y., Ma, P.F., Li, H.T., Yang, J.B., Wang, H. & Li, D.Z. (2016) Plastid phylogenomic analyses resolve Tofieldiaceae as the root of the early diverging monocot order Alismatales. Genome Biology and Evolution 8 (3): 932–945. https://doi.org/10.1093/gbe/evv260
Ma, J., Yang, B.X., Zhu, W., Sun, L.L., Tian, J.K. & Wang, X.M. (2013) The complete chloroplast genome sequence of Mahonia bealei (Berberidaceae) reveals a significant expansion of the inverted repeat and phylogenetic relationship with other angiosperms. Gene 528 (2): 120–131. https://doi.org/10.1016/j.gene.2013.07.037
Maximowicz, C.J. (1878) Diagnoses de nouvelles plantes asiatiques. ?. Bulletin de l’Acadeìmie Impeìriale des Sciences de St.-Peìtersbourg 24: 26–89.
Maximowicz, C.J. (1889) Flora Tangutica. 49 pp.
Maximowicz, C.J. (1889) In: Flora Tangutica. 49 T. 25 Figs. 1–5.
Matuda. (1950) In: Revista de la Sociedad Mexicana de Historia Natural 11: 91.
Mower, J.P. & Vickrey, T.L. (2018) Structural diversity among plastid genomes of land plants. Advances in Botanical Research 85: 263–292. https://doi.org/10.1016/bs.abr.2017.11.013
Palmer, J.D. & Thompson, W.F. (1982) Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell 29 (2): 537–550. https://doi.org/10.1016/0092-8674(82)90170-2
Palmer, J.D. (1983) Chloroplast DNA exists in two orientations. Nature 301 (5895): 92–93. https://doi.org/10.1038/301092a0
Park, S., An, B. & Park, S.J. (2018) Reconfiguration of the plastid genome in Lamprocapnos spectabilis: IR boundary shifting, inversion, and intraspecific variation. Scientific Reports 8 (1): 1–14. https://doi.org/10.1038/s41598-018-31938-w
Pérez-Gutiérrez, M.A., Romero-García, A.T., Fernández, M.C., Blanca, G., Salinas-Bonillo, M.J. & Suárez-Santiago, V.N. (2015) Evolutionary history of Fumitories (subfamily Fumarioideae, Papaveraceae): An old story shaped by the main geological and climatic events in the Northern Hemisphere. Molecular Phylogenetics and Evolution 88: 75–92. https://doi.org/10.1016/j.ympev.2015.03.026
Prain ex Fedde. (1925) In: Repertorium specierum novarum regni vegetabilis 22: 26.
Qi, W.K., Lin, F., Liu, Y.H., Huang, B.Q., Cheng, J.H., Zhang, W. & Zhao, H. (2016) High-throughput development of simple sequence repeat markers for genetic diversity research in Crambe abyssinica. BMC Plant Biology 16 (1): 1–11. https://doi.org/10.1186/s12870-016-0828-y
Qu, X.J., Moore, M.J., Li, D.Z. & Yi, T.S. (2019) PGA: A software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 15 (1): 1–12. https://doi.org/10.1186/s13007-019-0435-7
Ren, F.M., Wang, Y.W., Xu, Z.C., Li, Y., Xin, T.Y., Zhou, J.G., Qi, Y.D., Wei, X.P., Yao, H. & Song, J.Y. (2019) DNA barcoding of Corydalis, the most taxonomically complicated genus of Papaveraceae. Ecology and Evolution 9 (4): 1934–1945. https://doi.org/10.1002/ece3.4886
Ren, F.M., Wang, L.Q., Li, Y., Zhuo, W., Xu, Z.C., Guo, H., Liu, Y., Gao, R.R. & Song, J.Y. (2021) Highly variable chloroplast genome from two endangered Papaveraceae lithophytes Corydalis tomentella and Corydalis saxicola. Ecology and Evolution 11 (9): 4158–4171. https://doi.org/10.1002/ece3.7312
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012) Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61 (3): 539–542. https://doi.org/10.1093/sysbio/sys029
Ruhlman, T.A., Chang, W.J., Chen, J.J.W., Huang, Y.T., Chan, M.T., Zhang, J., Liao, D.C., Blazier, J.C., Jin, X., Shih, M.C., Jansen, R.K. & Lin, C.S. (2015) NDH expression marks major transitions in plant evolution and reveals coordinate intracellular gene loss. BMC Plant Biology 15 (1): 1–9. https://doi.org/10.1186/s12870-015-0484-7
Salie, M.J. & Thelen, J.J. (2016) Regulation and structure of the heteromeric acetyl-CoA carboxylase. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids 1861 (9): 1207–1213. https://doi.org/10.1016/j.bbalip.2016.04.004
Sauquet, H., Carrive, L., Poullain, N., Sannier, J., Damerval, C. & Nadot, S. (2015) Zygomorphy evolved from disymmetry in Fumarioideae (Papaveraceae, Ranunculales): New evidence from an expanded molecular phylogenetic framework. Annals of Botany 115 (6): 895–914. https://doi.org/10.1093/aob/mcv020
Stamatakis, A. (2014) RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30 (9): 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Sun, Y.X, Moore, M.J., Zhang, S., Soltis, P.S., Soltis, D.E., Zhao, T., Meng, A., Li, X., Li, J. & Wang, H.C. (2016) Phylogenomic and structural analyses of 18 complete plastomes across nearly all families of early-diverging eudicots, including an angiosperm-wide analysis of IR gene content evolution. Molecular Phylogenetics and Evolution 96: 93–101. https://doi.org/10.1016/j.ympev.2015.12.006
Sun, Y.X, Moore, M.J., Lin, N., Adelalu, K.F., Meng, A., Jian, S., Yang, L., Li, J. & Wang, H.C. (2017) Complete plastome sequencing of both living species of Circaeasteraceae (Ranunculales) reveals unusual rearrangements and the loss of the ndh gene family. BMC Genomics 18 (1): 1–10. https://doi.org/10.1186/s12864-017-3956-3
Suyama, M., Torrents, D. & Bork, P. (2006) PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Research 34 (Web Server): W609–W612. https://doi.org/10.1093/nar/gkl315
Su, Z.Y. (1986) Sect. Hamatae, one new section of Corydalis from China. Acta Botanica Yunnanica 8 (4): 407–412.
Thiel, T., Michalek, W., Varshney, R.K. & Graner, A. (2003) Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theoretical and Applied Genetics 106 (3): 411–422. https://doi.org/10.1007/s00122-002-1031-0
Walker, J.F., Jansen, R.K., Zanis, M.J. & Emery, N.C. (2015) Sources of inversion variation in the small single copy (SSC) region of chloroplast genomes. American Journal of Botany 102 (11): 1751–1752. https://doi.org/10.3732/ajb.1500299
Wang, D.P., Zhang, Y.B., Zhang, Z., Zhu, J. & Yu, J. (2010) KaKs_Calculator 2.0: A Toolkit Incorporating Gamma-Series Methods and Sliding Window Strategies. Genomics, Proteomics and Bioinformatics 8 (1): 77–80. https://doi.org/10.1016/S1672-0229(10)60008-3
Wang, T.P. (1934) In: Contributions from the Institute of Botany, National Academy of Peiping 2: 301.
Wang, Y.W. (2006) Systematics of Corydalis DC. (Fumariaceae). Institute of Botany, the Chinese Academy of Sciences, Beijing, 129 pp.
Wang, W.T. (1984) In: Fl. Beijing 1: 670.
Weng, M.L., Blazier, J.C., Govindu, M. & Jansen, R.K. (2014) Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Molecular Biology and Evolution 31 (3): 645–659. https://doi.org/10.1093/molbev/mst257
Willdenow, K.L. (1804) In: Species plantarum 3 (3): 1838.
Wu, C.S., Wang, Y.N., Liu, S.M. & Chaw, S.M. (2007) Chloroplast genome (cpDNA) of Cycas taitungensis and 56 cp protein-coding genes of Gnetum parvifolium: Insights into cpDNA evolution and phylogeny of extant seed plants. Molecular Biology and Evolution 24 (6): 1366–1379. https://doi.org/10.1093/molbev/msm059
Wu, J., Lin, P.C., Guo, Y.P. & Liu, M.D. (2020) The complete chloroplast genome of Corydalis conspersa. Mitochondrial DNA Part B: Resources 5 (2): 1977–1978. https://doi.org/10.1080/23802359.2020.1756944
Wu, Z.Y., Zhuang, X. & Su, Z.Y. (1996) The systematic evolution of Corydalis in relation to florogenesis and floristic regionalization in the world. Acta Botanica Yunnanica 18 (3): 241–267.
Wu, Z.Y., Zhuang, X. & Su, Z.Y. (1999) Corydalis DC. In: Wu, Z.Y. (Ed.) Florae Reipublicae Popularis Sinicae, vol. 32. Science Press, Beijing, China, pp. 106–479.
Wu, Z.Y., Zhuang, X. (1990) A New section of Corydalis, Sect. Davidianae. Acta Botanica Yunnanica 12 (3): 279–286.
Wu, S., Chen, J., Li, Y., Liu, A., Li, A., Yin, M., Shrestha, N., Liu, J. & Ren, G. (2021) Extensive genomic rearrangements mediated by repetitive sequences in plastomes of Medicago and its relatives. BMC Plant Biology 21 (1): 1–17. https://doi.org/10.1186/s12870-021-03202-3
Xi, Z., Rest, J.S. & Davis, C.C. (2013) Phylogenomics and coalescent analyses resolve extant seed plant relationships. PLoS ONE 8 (11): 21–24. https://doi.org/10.1371/journal.pone.0080870
Xia, M.Q., Liao, R.Y., Zhou, J.T., Lin, H.Y., Li, J.H., Li, P., Fu, C.X. & Qiu, Y.X. (2021) Phylogenomics and biogeography of Wisteria: Implications on plastome evolution among inverted repeat-lacking clade (IRLC) legumes. Journal of Systematics and Evolution 60 (2): 253–265. https://doi.org/10.1111/jse.12733
Xu, X.D. & Wang, D. (2018) Corydalis ternatifolia belongs to C. Sect. Asterostigmata, not C. sect. incisae (papaveraceae): Evidence from morphological and phylogenetic study. Phytotaxa 382 (2): 193–203. https://doi.org/10.11646/phytotaxa.382.2.4
Xu, X.D. & Wang, D. (2020) Characterization of the complete chloroplast genome of Corydalis inopinata Prain ex Fedde (Papaveraceae). Mitochondrial DNA Part B: Resources 5 (3): 3302–3303. https://doi.org/10.1080/23802359.2020.1814887
Xu, X.D. & Wang, D. (2021) Comparative Chloroplast Genomics of Corydalis Species (Papaveraceae): Evolutionary Perspectives on Their Unusual Large Scale Rearrangements. Frontiers in Plant Science 11: 600354. https://doi.org/10.3389/fpls.2020.600354
Yu, Z.Y., Zhou, T.H., Li, N.Y. & Wang, D. (2021) The complete chloroplast genome and phylogenetic analysis of Corydalis fangshanensis W. T. Wang ex S. Y. He (Papaveraceae). Mitochondrial DNA Part B: Resources 6 (11): 3171–3173. https://doi.org/10.1080/23802359.2021.1987172
Zhai, W., Duan, X.S., Zhang, R., Guo, C.C., Li, L., Xu, G.X., Shan, H.Y., Kong, H.Z. & Ren, Y. (2019) Chloroplast genomic data provide new and robust insights into the phylogeny and evolution of the Ranunculaceae. Molecular Phylogenetics and Evolution 135: 12–21. https://doi.org/10.1016/j.ympev.2019.02.024
Zhang, M.L., Su, Z.Y. & Lidén, M. (2008) Corydalis DC. In: Wu, Z.Y., Raven, P.H. & Hong, D.Y. (Ed.) Flora of China, vol. 7. Science Press, Beijing and Missouri Botanical Garden Press, St. Louis, pp. 295–427.
Zhang, Z.X., Wang, D. & Yang, X. (2016) The taxonomic position of Corydalis parviflora Su & Lidén (Papaveraceae), a genetically distinct species: Evidence from cpDNA and nDNA sequences. Biochemical Systematics and Ecology 67: 134–141. https://doi.org/10.1016/j.bse.2016.06.003
Zhou, T., Ruhsam, M., Wang, J., Zhu, H.H., Li, W.L., Zhang, X., Xu, Y.C., Xu, F.S. & Wang, X.M. (2019) The Complete Chloroplast Genome of Euphrasia regelii, Pseudogenization of ndh Genes and the Phylogenetic Relationships within Orobanchaceae. Frontiers in Genetics 10: 444. https://doi.org/10.3389/fgene.2019.00444