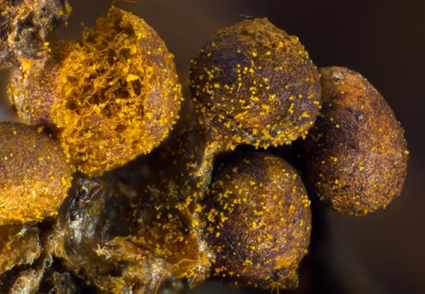Abstract
The Myxomycetes comprise a remarkably diverse group of organisms within Amoebozoa, with over 1000 species currently recognized. These organisms, at the end of their life cycles produce fruiting bodies which are the basis for their systematics. Despite being a biodiversity hotspot, the tropical Andes has a myxobiota that remains barely explored so far. In this study, we report the occurrence of three species inhabiting the highlands of the Peruvian Andes between 3000–5000 m.a.s.l. Arcyodes incarnata and Trichia mirabilis are reported for the first time in the Southern Hemisphere and the Neotropics, respectively, while Metatrichia floripara represents the third record in the world, previously only known from Rwanda and Brazil. Fruiting bodies of T. mirabilis were detected as already developed in the field, while the previous reports only included moist chamber culture-derived specimens. These results notably enlarge the geographical distribution of these species and highlight the interest of the tropical Andes, which remains a challenging region to explore and determine its whole myxobiota.
References
Bortnikov, F.M., Matveev, A.V., Gmoshinskiy, V.I., Novozhilov, Yu.K., Zemlyanskaya, I.V., Vlasenko, A.V., Schnittler, M., Shchepin, O.N. & Fedorova, N.A. (2020) Myxomycetes of Russia: a history of research and a checklist of species. Karstenia 58 (2): 316–373. https://doi.org/10.29203/ka.2020.502
Clissmann, F., Fiore-Donno, A.M., Hoppe, B., Krüger, D., Kahl, T., Unterseher, M. & Schnittler, M. (2015) First insight into dead wood protistan diversity: a molecular sampling of bright-spored Myxomycetes (Amoebozoa, slime-moulds) in decaying beech logs. FEMS Microbiology Ecology 91 (6). https://doi.org/10.1093/femsec/fiv050
Dagamac, N.H.A., Novozhilov, Y.K., Stephenson, S.L., Lado, C., Rojas, C., dela Cruz, T.E.E., Unterseher, M. & Schnittler, M. (2017) Biogeographical assessment of myxomycete assemblages from Neotropical and Asian Palaeotropical forests. Journal of Biogeography 44: 1524–1536. https://doi.org/10.1111/jbi.12985
De Lima, V.X. & Cavalcanti, L.D.H. (2016) Second world record of Metatrichia floripara (Trichiaceae, Myxomycetes), found in Brazil. Phytotaxa 247: 292–294. https://doi.org/10.11646/phytotaxa.247.4.8
Estrada-Torres, A., Wrigley de Basanta, D., Conde, E. & Lado, C. (2009) Myxomycetes associated with dryland ecosystems of the Tehuacán-Cuicatlán Valley Biosphere Reserve, Mexico. Fungal Diversity 36: 17–56.
Farr, M.L. (1976) Flora Neotropica. Monograph No. 16. Myxomycetes. The New York Botanical Garden, New York. 304 pp.
Fiore-Donno, A.M., Clissmann, F., Meyer, M., Schnittler, M. & Cavalier-Smith, T. (2013) Two-gene phylogeny of bright-spored Myxomycetes (slime moulds, Superorder Lucisporidia). S. Gribaldo (ed.) PLoS ONE 8: e62586. https://doi.org/10.1371/journal.pone.0062586
GBIF.org (2021) Occurrence dataset. Available from: https://doi.org/10.15468/dl.mpsfxj (accessed 21 June 2021).
Hoppe, T. & Schnittler, M. (2015) Characterization of Myxomycetes in two different soils by TRFLP-analysis of partial 18S rRNA gene sequences. Mycosphere 6: 216–227. https://doi.org/10.5943/mycosphere/6/1/5
Kamono, A., Meyer, M., Cavalier-Smith, T., Fukui, M. & Fiore-Donno, A.M. (2013) Exploring slime mould diversity in high-altitude forests and grasslands by environmental RNA analysis. FEMS Microbiology Ecology 84: 98–109. https://doi.org/10.1111/1574-6941.12042
Kelly, K.L. & Judd, D.B. (1976) ISCC-NBS Color Name Charts Illustrated with Centroid Colors. Inter-Society Color Council. National Bureau of Standards, Washington.
Lado, C. (2005–2021) An on line nomenclatural information system of Eumycetozoa. Available from: http://www.nomen.eumycetozoa.com (Last updated 31 May 2021)
Lado, C. & Pando, F. (1997) Flora Mycologica Iberica, Vol. 2. Myxomycetes, I. Ceratiomyxales, Echinosteliales, Liceales, Trichiales. J. Cramer. 323 pp.
Lado, C. & Rojas, C. (2020) Guía para el estudio de la taxonomía y ecología de Myxomycetes. Tecnoprint, San Jose.
Lado, C., Wrigley de Basanta, D. & Estrada-Torres, A. (2011) Biodiversity of Myxomycetes from the Monte Desert of Argentina. Anales del Jardín Botánico de Madrid 68: 61–95. https://doi.org/10.3989/ajbm.2266
Lado, C., Wrigley de Basanta, D., Estrada-Torres, A. & Stephenson, S.L. (2013) The biodiversity of myxomycetes in central Chile. Fungal Diversity 59: 3–32. https://doi.org/10.1007/s13225-012-0159-8
Lado, C., Wrigley de Basanta, D., Estrada-Torres, A. & García-Carvajal, E. (2014) Myxomycete diversity of the Patagonian Steppe and bordering areas in Argentina. Anales del Jardín Botánico de Madrid 71: e006. https://doi.org/10.3989/ajbm.2394
Lado, C., Wrigley de Basanta, D., Estrada-Torres, A. & Stephenson, S.L. (2016) Myxomycete diversity in the coastal desert of Peru with emphasis on the lomas formations. Anales del Jardín Botánico de Madrid 73: e032. https://doi.org/10.3989/ajbm.2436
Lado, C., Estrada-Torres, A. & Rojas Alvarado, C. (2018) New records of genera and species of myxomycetes (Amoebozoa) from the Neotropics. Check List 14: 509–518. https://doi.org/10.15560/14.3.509
Lado, C., Wrigley de Basanta, D., Estrada-Torres, A., Stephenson, S.L. & Treviño, I. (2019) Diversity of Myxomycetes in arid zones of Peru part II: the cactus belt and transition zones. Anales del Jardín Botánico de Madrid 76: 083. https://doi.org/10.3989/ajbm.2520
Lara, E., Dumack, K., García-Martín, J.M., Kudryavtsev, A. & Kosakyan, A. (2020) Amoeboid protist systematics: a report on the “Systematics of amoeboid protists” symposium at the VIIIth ECOP/ISOP meeting in Rome, 2019. European Journal of Protistology 76: 125727. https://doi.org/10.1016/j.ejop.2020.125727
Leontyev, D., McHugh, R., Fefelov, K.A. & Kochergina, A.V. (2011) New and rare Myxomycetes of Ukraine. 2. Southwest Crimea. Nova Hedwigia 92: 245–256.
Liu, Q.-S., Yan, S.-Z. & Chen, S.-L. (2015) Species diversity of myxomycetes associated with different terrestrial ecosystems, substrata (microhabitats) and environmental factors. Mycological Progress 14: 27. https://doi.org/10.1007/s11557-015-1048-9
Martin, G.W. & Alexopoulos, C.J. (1969) The Myxomycetes. University of Iowa Press, Iowa. 560 pp.
Muñoz, J., Felicísimo, A.M., Cabezas, F., Burgaz, A.R. & Martínez, I. (2004) Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science 304: 1144-1147. https://doi.org/10.1126/science.1095210
Nannenga-Bremekamp, N.E. (1966) Notes on Myxomycetes X. Some new species of Licea, Reticularia, Cribraria, Dictydiaethalium, Trichia and Metatrichia. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen 69: 336–349.
Nannenga-Bremekamp, N.E. (1985) Notes on Myxomycetes. XXII. Three new species, two new families and four new combinations. Proceedings Van De Koninklijke Nederlandse Akademie van Wetenschappen, Section C 88 (1): 121–128.
Nannenga-Bremekamp, N.E. (1991) A Guide to Temperate Myxomycetes. Biopress. 428 pp.
Ndiritu, G.G., Spiegel, F.W. & Stephenson, S.L. (2009a) Distribution and ecology of the assemblages of myxomycetes associated with major vegetation types in Big Bend National Park, USA. Fungal Ecology 2: 168–183. https://doi.org/10.1016/j.funeco.2009.03.002
Ndiritu, G.G., Winsett, K.E., Spiegel, F.W. & Stephenson, S.L. (2009b) A checklist of African myxomycetes. Mycotaxon 107: 353–356. https://doi.org/10.5248/107.353
Novozhilov, Y.K., Schnittler, M., Vlasenko, A.V. & Fefelov, K.A. (2010) Myxomycete diversity of the Altay Mountains (southwestern Siberia, Russia). Mycotaxon 111: 91–94.
Novozhilov, Y.K., Erastova, D.A., Shchepin, O.N., Schnittler, M., Alexandrova, A.V., Popov, E.S. & Kuznetov, A.N. (2017a) Myxomycetes associated with monsoon lowland tropical forests in southern Vietnam. Nova Hedwigia 104: 143–182. https://doi.org/10.1127/nova_hedwigia/2016/0395
Novozhilov, Y.K., Rollins, A.W. & Schnittler, M. (2017b) Ecology and distribution of Myxomycetes. Myxomycetes. Biology, Systematics, Biogeography, and Ecology. Elsevier, pp. 253–297.
Novozhilov, Y.K., Schnittler, M., Erastova, D.A. & Shchepin, O.N. (2017c) Myxomycetes of the Sikhote-Alin State Nature Biosphere Reserve (Far East, Russia). Nova Hedwigia 104: 183–209. https://doi.org/10.1127/nova_hedwigia/2016/0394
Nyirambangutse, B., Zibera, E., Uwizeye, F.K., Nsabimana, D., Bizuru, E., Pleijel, H., Uddling, J. & Wallin, G. (2017) Carbon stocks and dynamics at different successional stages in an Afromontane tropical forest. Biogeosciences 14: 1285–1303. https://doi.org/10.5194/bg-14-1285-2017
Poulain, M., Meyer, M. & Bozonnet, J. (2011) Les Myxomycètes. Fédération mycologique et botanique Dauphiné-Savoie. Sevrier Pl 215. 406 pp.
Rammeloo, J. (1981) Five new Myxomycete species (Trichiales) from Rwanda. Bulletin du Jardin botanique national de Belgique 51: 229–230. https://doi.org/10.2307/3667752
Ranade, V.D., Korade, S.T., Jagtap, A.V. & Ranadive, K.R. (2012) Checklist of Myxomycetes from India. Mycosphere 3: 358–390. https://doi.org/10.5943/mycosphere/3/3/9
Rojas, C., Stephenson, S.L. & Pavlich, M. (2011) New additions to the myxobiota of Peru. Mycosphere 2: 583–592. https://doi.org/10.5943/mycosphere/2/5/8
Rojas, C., Stephenson, S.L., Valverde, R. & Estrada-Torres, A. (2012) A biogeographical evaluation of high-elevation myxomycete assemblages in the northern Neotropics. Fungal Ecology 5: 99–113. https://doi.org/10.1016/j.funeco.2011.06.005
Rollins, A.W. & Stephenson, S.L. (2011) Global distribution and ecology of myxomycetes. Current Topics in Plant Biology 12: 1–14.
Rostafi?ski, J.T. (1875) ?luzowce (Mycetozoa) monografia. Nak?adem Biblioteki Kórnickiej. 524 pp.
Schnittler, M. & Mitchell, D.W. (2000) Species diversity in Myxomycetes based on the morphological species concept. A critical examination. Stapfia 73: 55–62.
Schnittler, M., Dagamac, N.H.A. & Novozhilov, Y.K. (2017) Biogeographical patterns in Myxomycetes. Myxomycetes. Biology, Systematics, Biogeography, and Ecology. Elsevier, pp. 299–331.
Shchepin, O., Schnittler, M., Dagamac, N., Leontyev, D. & Novozhilov, Y. (2019) Unexplored diversity of microscopic myxomycetes: evidence from environmental DNA. Plant Ecology and Evolution 152: 499–506. https://doi.org/10.5091/plecevo.2019.1621
Spegazzini, C. (1921) Mycetes Chilensis. Boletin de la Academia Nacional de Ciencias en Córdoba 25: 1–124.
Stephenson, S.L., Schnittler, M. & Novozhilov, Y.K. (2008) Myxomycete diversity and distribution from the fossil record to the present. Biodiversity and Conservation 17: 285–301. https://doi.org/10.1007/s10531-007-9252-9
Stephenson, S.L., Wrigley de Basanta, D., Lado, C., Estrada-Torres, A. & Darrah, R. (2019) Myxomycete biodiversity revealed in the Namib desert. South African Journal of Botany 124: 402–413. https://doi.org/10.1016/j.sajb.2019.06.002
Treviño-Zevallos, I.F. & Lado Rodriguez, C. (2020) New records of Myxomycetes from Peru. Check List 16: 253–264. https://doi.org/10.15560/16.2.253
Wrigley de Basanta, D. & Estrada-Torres, A. (2017) Techniques for recording and isolating Myxomycetes. In: Stephenson, S.L. & Rojas, C. (Eds.) Myxomycetes. Biology, Systematics, Biogeography, and Ecology. Elsevier, pp. 333–363.
Yamamoto, Y. (2021) Biota of Japanese Myxomycetes. Committee for the publication of “Biota of Japanese Myxomycetes”, Ibaraki, 1136 pp.
Zhang, B. & Li, Y. (2013) Myxomycetes from China 16: Arcyodes incarnata and Licea retiformis, newly recorded for China. Mycotaxon 122: 157–160. https://doi.org/10.5248/122.157