Abstract
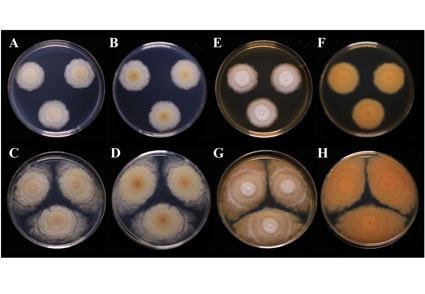
A strain of the fungal genus Mariannaea, isolated from decayed leaves of Japanese red pine (Pinus densiflora), was identified to be a new species based on its molecular phylogeny and morphology. Molecular phylogenetic analysis revealed that the fungus belongs to a clade containing M. punicea and related species (M. punicea-related species) and that it forms a lineage independent of these species. Microscopic morphological comparisons with M. punicea-related species indicated that the new isolate differs with respect to the width of the phialides and length of the conidiophores. Comparison of macroscopic morphological characteristics revealed that M. punicea-related species are characterized by reddish-purple colonies, whereas the new isolate lacks this distinctive pigmentation. Moreover, the surface structure of the colonies of this fungus has a distinct irregular undulate pattern toward the margins. Given that this isolate can be clearly distinguished from the known M. punicea-related species, we consider the fungus to be a newly identified species, for which we propose the name Mariannaea imbricata sp. nov.
References
Crous, P.W., Wingfield, M.J., Lombard, L., Roets, F., Swart, W.J., Alvarado, P., Carnegie, A.J., Moreno, G., Luangsa-Ard, J., Thangavel, R., Alexandrova, A.V., Baseia, I.G., Bellanger, J.M., Bessette, A.E., Bessette, A.R., Delapeña-Lastra, S., García, D., Gené, J., Pham, T.H.G., Heykoop, M., Malysheva, E., Malysheva, V., Martín, M.P., Morozova, O.V., Noisripoom, W., Overton, B.E., Rea, A.E., Sewall, B.J., Smith, M.E., Smyth, C.W., Tasanathai, K., Visagie, C.M., Adam?ík, S., Alves, A., Andrade, J.P., Aninat, M.J., Araújo, R.V.B., Bordallo, J.J., Boufleur, T., Baroncelli, R., Barreto, R.W., Bolin, J., Cabero, J., Cabo?, M., Cafà, G., Caffot, M.L.H., Cai, L., Carlavilla, J.R., Chávez, R., Decastro, R.R.L., Delgat, L., Deschuyteneer, D., Dios, M.M., Domínguez, L.S., Evans, H.C., Eyssartier, G., Ferreira, B.W., Figueiredo, C.N., Liu, F., Fournier, J., Galli-Terasawa, L.V., Gil-Durán, C., Glienke, C., Gonçalves, M.F.M., Gryta, H., Guarro, J., Himaman, W., Hywel-Jones, N., Iturrieta-González, I., Ivanushkina, N.E., Jargeat, P., Khalid, A.N., Khan, J., Kiran, M., Kiss, L., Kochkina, G.A., Kola?ík, M., Kubátová, A., Lodge, D.J., Loizides, M., Luque, D., Manjón, J.L., Marbach, P.A.S., Massolajr, N.S., Mata, M., Miller, A.N., Mongkolsamrit, S., Moreau, P.A., Morte, A., Mujic, A., Navarro-Ródenas, A., Németh, M.Z., Nóbrega, T.F., Nováková, A., Olariaga, I., Ozerskaya, S.M., Palma, M.A., Petters-Vandresen, D.A.L., Piontelli, E., Popov, E.S., Rodríguez, A., Requejo, Ó., Rodrigues, A.C.M., Rong, I.H., Roux, J., Seifert, K.A., Silva, B.D.B., Sklená?, F., Smith, J.A., Sousa, J.O., Souza, H.G., Desouza, J.T., Švec, K., Tanchaud, P., Tanney, J.B., Terasawa, F., Thanakitpipattana, D., Torres-Garcia, D., Vaca, I., Vaghefi, N., Vaniperen, A.L., Vasilenko, O.V., Verbeken, A., Yilmaz, N., Zamora, J.C., Zapata, M., Jurjevi, Ž. & Groenewald, J.Z. (2019) Fungal Planet description sheets: 868–950. Persoonia 42: 291–473. https://doi.org/10.3767/persoonia
Darriba, D., Posada, D., Kozlov, A.M., Stamatakis, A., Morel, B. & Flouri, T. (2020) ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution 37: 291–294. https://doi.org/10.1093/molbev/msz189
Domsch, K.H., Grams, W. & Anderson, T.H. (2007) Compendium of soil fungi second edition. pp. 256–266.
Edler, D., Klein, J., Antonelli, A. & Silvestro, D. (2021) raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods in Ecology and Evolution 12: 373–377. https://doi.org/10.1111/2041-210X.13512
Fukuda, T., Sudoh, Y., Tsuchiya, Y., Okuda, T., Fujimori, F. & Igarashi, Y. (2011) Marianins A and B, prenylated phenylpropanoids from Mariannaea camptospora. Journal of Natural Products 74: 1327–1330. https://doi.org/10.1021/np200035m
Glass, N.L. & Donaldson, G.C. (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Gräfenhan, T., Schroers, H.J., Nirenberg, H.I. & Seifert, K.A. (2011) An overview of the taxonomy, phylogeny and typification of some nectriaceous fungi classified in Cosmospora, Acremonium, Fusarium, Stilbella and Volutella. Studies in Mycology 68: 79–113. https://doi.org/10.3114/sim.2011.68.04
Hu, D.H., Wang, M. & Cai, L. (2017) Phylogenetic assessment and taxonomic revision of Mariannaea. Mycological Progress 16: 271–283. https://doi.org/10.1007/s11557-016-1252-2
Hyde, K.D., Dong, Y., Phookamsak, R., Jeewon, R., Bhat, D.J., Jones, E.B.G., Liu, N.G., Abeywickrama, P.D., Mapook, A., Wei, D., Perera, R.H., Manawasinghe, I.S., Pem, D., Bundhun, D., Karunarathna, A., Ekanayaka, A.H., Bao, D.F., Li, J., Samarakoon, M.C., Chaiwan, N., Lin, C.G., Phutthacharoen, K., Zhang, S.N., Senanayake, I.C., Goonasekara, I.D., Thambugala, K.M., Phukhamsakda, C., Tennakoon, D.S., Jiang, H.B., Yang, J., Zeng, M., Huanraluek, N., Liu, J.K., Wijesinghe, S.N., Tian, Q., Tibpromma, S., Brahmanage, R.S., Boonmee, S., Huang, S.K., Thiyagaraja, V., Lu, Y.Z., Jayawardena, R.S., Dong, W., Yang, E.F., Singh, S.K., Singh, S.M., Rana, S., Lad, S.S., Anand, G., Devadatha, B., Niranjan, M., Sarma, V.V., Liimatainen, K., Aguirre-Hudson, B., Niskanen, T., Overall, A., Alvarenga, R.L.M., Gibertoni, T.B., Pfliegler, W.P., Horváth, E., Imre, A., Alves, A.L., da Silva Santos, A.C., Tiago, P.V., Bulgakov, T.S., Wanasinghe, D.N., Bahkali, A.H., Doilom, M., Elgorban, A.M., Maharachchikumbura, S.S.N., Rajeshkumar, K.C., Haelewaters, D., Mortimer, P.E., Zhao, Q., Lumyong, S., Xu, J. & Sheng, J. (2020) Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity 100: 5–277. https://doi.org/10.1007/s13225-020-00439-5
Ishiuchi, K., Hirose, D., Kondo., Watanabe, K., Terasaka, K. & Makino, T. (2020) Mariannamides A and B, new cyclic octapeptides isolated from Mariannaea elegans NBRC102301. Bioorganic & Medicinal Chemistry Letters 30: 126946. https://doi.org/10.1016/j.bmcl.2019.126946
Katoh, K., Rozewicki, J. & Yamada, K.D. (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. https://doi.org/10.1093/bib/bbx108
Kozlov, A.M., Darriba, D., Flouri, T., Morel, B. & Stamatakis, A. (2019) RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35: 4453–4455. https://doi.org/10.1093/bioinformatics/btz305
Lemoine, F., Domelevo Entfellner, J.B., Wilkinson, E., Correia, D., Dávila Felipe, M., De Oliveira, T. & Gascuel, O. (2018) Renewing Felsenstein’s phylogenetic bootstrap in the era of big data. Nature 556: 452–456. https://doi.org/10.1038/s41586-018-0043-0
Lombard, L., Van der Merwe, N.A., Groenewald, J.Z. & Crous, P.W. (2015) Generic concepts in Nectriaceae. Studies in Mycology 80: 189–245. http://dx.doi.org/10.1016/j.simyco.2014.12.002
Luangsa-ard, J.J., Hywel-Jones, N.L., Manoch, L. & Samson, R.A. (2005) On the relationships of Paecilomyces sect. Isarioidea species. Mycological Research 109 (05): 581–589. https://doi.org/10.1017/S0953756205002741
Matsushima, T. (1995) Matsushima Mycological Memoirs No. 8. Matsushima Fungus Collect, Kobe, Japan Murray, M.G. & Thompson, W.F. (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research 8: 4321–4326. https://doi.org/10.1093/nar/8.19.4321
Nonaka, K., Kaneta, T., Omura, S. & Masuma, R. (2015) Mariannaea macrochlamydospora, a new hyphomycete (Nectriaceae) from soil in the Bonin Islands, Japan. Mycoscience 56: 29–33. http://dx.doi.org/10.1016/j.myc.2014.02.001
O’Donnell, K. & Cigelink, E. (1997) Two divergent intragenomic rRNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. https://doi.org/10.1006/mpev.1996.0376
Ogawa, Y., Suda, A., Kusama-Eguchi, K., Watanabe, K. & Tokumasu, S. (2005) Intraspecific groups of Umbelopsis ramanniana inferred from nucleotide sequences of nuclear rDNA internal transcribed spacer regions and sporangiospore morphology. Mycoscience 46: 343–351. http://dx.doi.org/10.1007/s10267-005-0257-5
Okuda, T. & Yamamoto, K. (2000) Materials for the fungus flora of Japan (56) Mariannaea camptospora and M. elegans var. punicea from Japan. Mycoscience 41: 411–414. https://doi.org/10.1007/BF02463957
Samson, R.A. (1974) Paecilomyces and some allied hyphomycetes. Studies in Mycology 6: 1–117.
Samson, R.A. & Bigg, W.L. (1988) A new species of Mariannaea from California. Mycologia 80 (1): 131–134. https://doi.org/10.1080/00275514.1988.12025512
Samuels, G.J. (1989) Nectria and Penicillifer. Mycologia 81 (3): 347–355. https://doi.org/10.1080/00275514.1989.12025758
Shimazu, M. & Sato, H. (1996) Media for selective isolation of an entomogenous fungus, Beauveria bassiana (Deuteromycotina: Hyphomycetes). Applied Entomology and Zoology 31 (2): 291–298. https://doi.org/10.1303/aez.31.291
Tokumasu, S. (1996) Mycofloral succession on Pinus densiflora needles on a modern site. Mycoscience 37: 313–321. https://doi.org/10.1007/BF02461303
Vu, D., Groenewald, M., de Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., Al-Hatmi, A., Groenewald, J.Z., Cardinali, G., Houbraken, J., Boekhout, T., Crous, P.W., Robert, V. & Verkley, G.J.M. (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. https://doi.org/10.1016/j.simyco.2018.05.001
White, T.J., Bruns, T., Lee, S. & Taylor, J.W. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J. & White, Y.J. (eds.) PCR Protocols: A Guide to Methods and Application. Academic Press, San Diego, pp. 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Zeng, Z.Q. & Zhuang, W.Z. (2014) A new holomorphic species of Mariannaea and epitypification of M. samuelsii. Mycological Progress 13: 967–973. https://doi.org/10.1007/s11557-014-0980-4